1

Tic40 | Inner envelope membrane translocon complex protein (chloroplast)
AS10 709 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A.thaliana , N. benthamiana, N. tabacum, C. roseus, O. sativa, P. patens, S. lycopersicum, T. aestivum, V. vinifera
compartment marker of chloroplast inner envelope membrane
- Product Info
-
Immunogen: KLH-conjugated synthetic peptide derived from available plant sequences of Tic40 including Arabidopsis thaliana UniProt: Q9FMD5, TAIR: At5g16620
Host: Rabbit Clonality: Polyclonal Purity: Serum Format: Lyophilized Quantity: 50 �l Reconstitution: For reconstitution please add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20�C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Immunofluorescence (IF), Western blot (WB) Recommended dilution: 1 : 2500 (WB) Expected | apparent MW: 48 | 45 kDa (Arabidopsis thaliana)
- Reactivity
-
Confirmed reactivity: Arabidosps thaliana, Catharantus roseus, Nicotiana benthamiana, Nicotiana tabacum, Oryza sativa, Physcomitrium patens, Solanum lycopersicum, Triticum aestivum, Zea mays, Vitis vinifera Predicted reactivity: Lactuca sativa, Picea sitchenis, Pisum sativum, Populus trichocarpa, Ricinus communis
Species of your interest not listed? Contact usNot reactive in: Chlamydomonas reinhardtii
- Application Examples
-
Application example 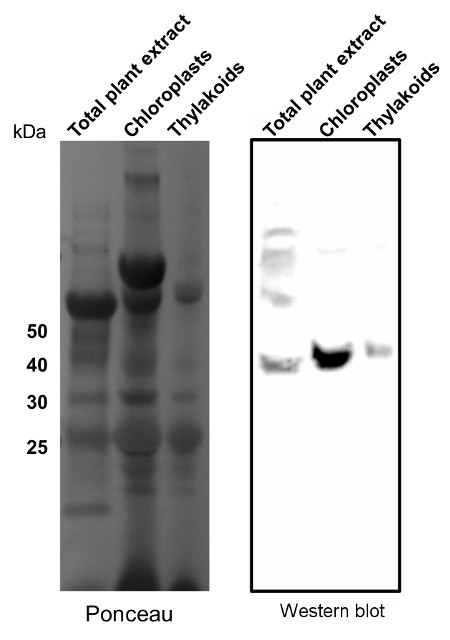
10-15µg of chlorophyll from isolated total plant material (Arabidopsis thaliana), chloroplasts and thylakoids extracted with a buffer containing (25 mM Tricine-NaOH, pH 7.8, 330 mM sorbitol, 1 mM EDTA, 10 mM KCl, 0.15% [w/v] bovine serum albumin, 4 mM sodium ascorbate, and 7 mM L-Cys) were separated on 12 % SDS-PAGE and blotted 1h to PVDF using semi-dry transfer. Blots were blocked with 10% milk for 1h at room temperature (RT) with agitation. Blot was incubated in the primary antibody at a dilution of 1: 2 000 overnight at 4⁰C with agitation. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 15 min and 3 times for 5 min in TBS-T at RT with agitation. Blot was incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, from Agrisera AS09 602) diluted to 1:15 000 in TBS-T for 1h at RT with agitation. The blot was washed as above and developed for 60 seconds with a ImageQuant system from GE Healthcare, exposure time was 60 seconds.
Courtesy of Dr. Rikard Fristedt VU University Amsterdam Faculty of Sciences Department of Physics and Astronomy Biophysics of Photosynthesis, The Netherlands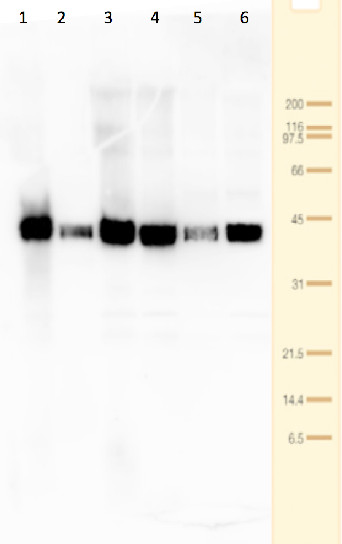
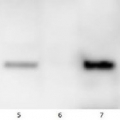
Lanes 1-6: 20μg 21kxg pellet from whole Physcomitrella patens protonemata extract (1), 20μg 21kxg supernatant from whole protonemata extract (2), 20μg broken chloroplasts/thylakoids from 40% Percoll band (3), 20μg intact chloroplasts from 80% Percoll band (4), 10μg pellet material from Percoll gradient (including whole cells and enriched for nuclei) (5), 20μg whole protonemata extract (6)
Sample Preparation Up to 20µg of total protein/chloroplast material from P. patens was prepared by chopping the protonemata into an ice cold isotonic chloroplast buffer (0.3M D-sorbitol, 50mM Hepes-KOH pH8.0, 2mM EDTA, 1mM MgCl2 with additives: 0.1% BSA and PVP-10 and added protease inhibitor tablet). The extract was filtered through a 70μm filter and chloroplasts were pelleted at 250 xg 4°C 5 min then layered onto discontinuous 10, 40, 80% Percoll gradients (which employed the same buffer) and centrifuged at 9k xg 4°C 1h. Broken chloroplasts/thylakoids and chloroplasts, respectively, were recovered from the 40 and 80% bands after centrifugation at 9k xg 4°C 1h by centrifugation at 1k xg in four volumes of buffer (without additives). Samples were frozen at -20°C. Samples were thawed on ice and were diluted in 50mM Tricine pH7.6, 1mM β-mercaptoethanol, 1mM MgCl2 with protease inhibitor tablet for protein concentration determinations and aliquots of up to 20 µg were prepared in 0.8 x Laemmli sample buffer and denatured at 95°C 5 min and were separated on 10-20% Criterion stain free SDS-PAGE and blotted 7 min to PVDF using BioRAD mini Turbo blot semi-dry transfer. Blots were blocked O/N at 4°C with agitation in 10% milk powder in PBS. Blot was rinsed in PBS-T 3 times for 5 min thenincubated in the primary antibody at a dilution of 1: 1 000 for anti-Toc75 or 1 in 2000 for antiTic40 for 2h at RT with agitation in 0.5% milk powder in PBS-T. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 15 min and 3 times for 5 min in PBS-T at RT with agitation. Blot was incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, from an unknown source but labelled NIF824) diluted to 1:2,500 in for 1h at RT with agitation in 0.5% milk powder in PBS-T. The blot was washed as above and developed for 10min with chemiluminescent detection reagent. Exposure time was 30 min.
Dr. Amanda Dowson, University of Warwick, UKApplication examples: 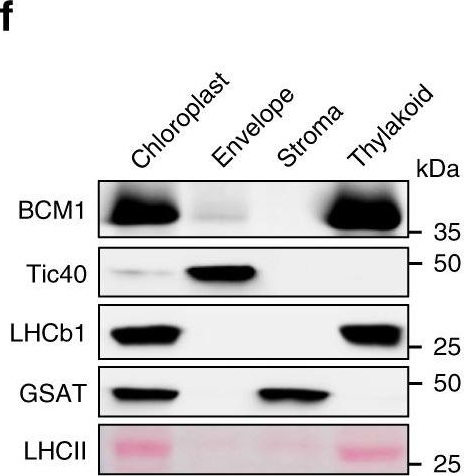
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 32198392
Journal: Nat Commun
Figure Number: 1F
Published Date: 2020-03-20
First Author: Wang, P., Richter, A. S., et al.
Impact Factor: 13.783
Open PublicationCharacterization of BCM1.a Co-expression analysis of BCM1. BCM1 together with the CBGs, GLK2, the carotenoid biosynthesis gene DEOXYXYLULOSE-5-PHOSPHATE SYNTHASE (DXS), and the gene encoding the chloroplast protein HIGH CHLOROPHYLL FLUORESCENT 107 (HCF107) were hierarchically clustered based on pairwise levels of similarity between their expression profiles, using the gene co-expression database ATTEDII (http://atted.jp/)76. Low distance values indicate high degrees of co-expression. b Accumulation of BCM1, CBEs, and LHCb1 in different organs of Arabidopsis. c Light-induced accumulation of BCM1, CBEs, and LHCb1 in 5-day-old etiolated WT seedlings, following illumination (80??mol photons?m?2?s?1) for 0, 3, 6, 12, and 24?h. d Schematic overview of the domain structure of BCM1. The cTP is shown in gray, while the six predicted TMDs are shown in red. e Subcellular localization of the BCM1-YFP fusion protein. Both BCM1-YFP and cTPBCM1-YFP fluorescence coincides with Chl autofluorescence, confirming chloroplast targeting of BCM1. In the control, YFP itself accumulates in the cytosol and nucleus. Scale bars, 5??m. f Suborganellar localization of BCM1. Chloroplasts from WT seedlings were subfractionated into envelope, stroma, and thylakoid fractions. For comparison, the proteins TRANSLOCON AT THE INNER ENVELOPE MEMBRANE OF CHLOROPLAST 40 (Tic40), GSAT, and LHC were specifically located in the envelope, stroma, and thylakoid, respectively. g Distribution of BCM1 across the different thylakoid membrane regions. Thylakoid proteins were solubilized with digitonin and fractionated into grana core, grana margins, and stroma lamellae. h Salt washing of thylakoid membranes. The WT thylakoids were sonicated in the presence of 1?M NaCl, 200?mM Na2CO3, 0.1?M NaOH, and 6?M urea on ice for 30?min before centrifugation to separate membrane (P) from soluble (S) fractions. Untreated thylakoids served as the control. LHCb1 and the CF1 ? subunit of ATP synthase, representing an intrinsic membrane protein and a peripheral thylakoid-associated protein, respectively, were used as positive controls for binding affinity to thylakoid membrane fractions. In b, c, f–h, immunoblot analyses were conducted using the indicated antibodies. Ponceau S-stained membrane strips bearing the large subunit of Rubisco (RbcL) or LHC proteins of PSII (LHCII) were used as loading controls.
Reactant: Triticum aestivum (Common wheat)
Application: Western Blotting
Pudmed ID: 33975629
Journal: Genome Biol
Figure Number: 5F
Published Date: 2021-05-11
First Author: Loudya, N., Mishra, P., et al.
Impact Factor: 13.214
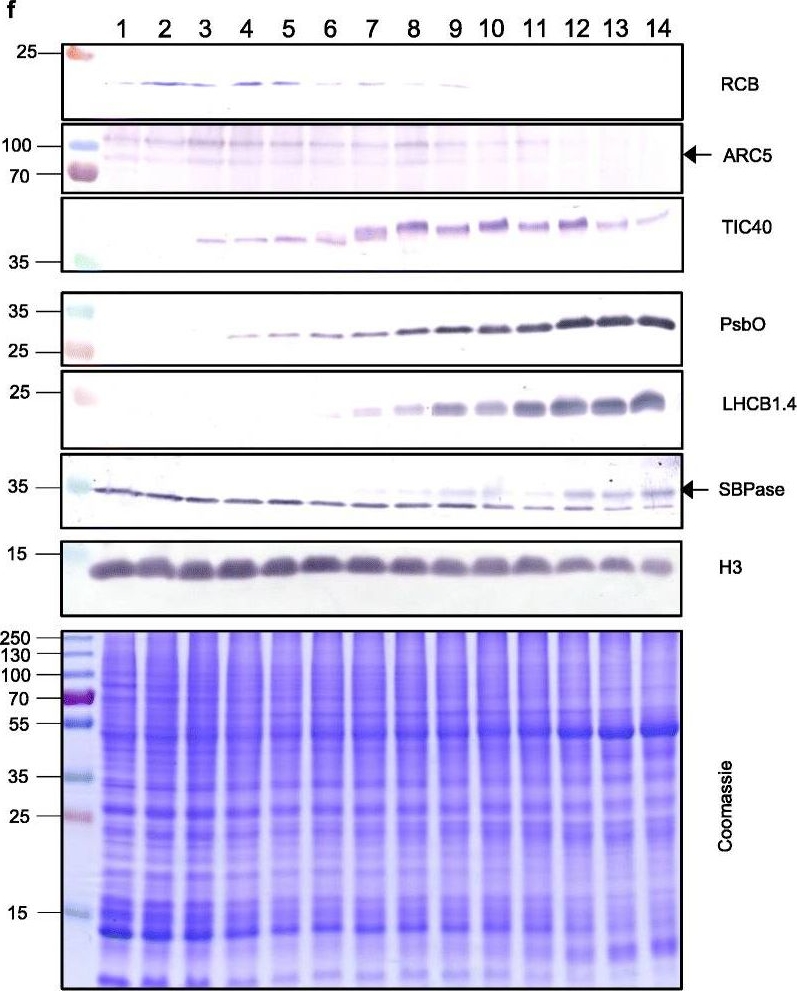
Open PublicationThe second, chloroplast growth phase involves greening and is supported by protein accumulation profiles. a Chlorophyll content, quantified in each of three independent biological replicates per sample. Error bars represent standard error of the mean. See Additional file 5: Table S6 for calculations. b, c, d Expression (Z-scores) of pigment biosynthesis and thylakoid biogenesis (b), light reactions (c) and carbon fixation-associated genes (d). e Expression (Z-scores) of chloroplast development-associated transcripts reflecting two stages of plastid development, peaking in the early plastid phase (RCB, ARC5 and TIC40) and, second, chloroplast phase (PSBO2, LHCB1.4 and SBPAse). f Immunoblot analysis of the protein products of the genes displayed in e. In total, 20??g of protein of samples 1–14 (for PSBO2, LHCB1.4 and SBPAse), 40??g (for RCB, ARC5 and TIC40) or 10??g (for Histone H3 as a constitutive control) was separated on denaturing SDS-PAGE gels, transferred to blots and probed with antibodies against the protein indicated. A Coomassie-stained total protein replica gel is also shown. Molecular weights (KDa) are indicated on the left. The results show one typical example from among three independent protein extraction and immunoblot experiments
- Additional Information
-
Additional information: Cellular [complartment marker] of chloroplast membrane Additional information (application): This product can be sold containing ProClin if requested
- Background
-
Background: Tic40 is a component of the inner envelope membrane import complex (TIC) of plant chloroplasts. Tic40 has been proposed to function as a co-chaperone in the stromal chaperone complex that facilitates protein translocation across the inner membrane. Tic40 can be used as a cellular [compartment marker] for chloroplast inner envelope membrane.
- Product Citations
-
Selected references: Loudya et al. (2021) Cellular and transcriptomic analyses reveal two-staged chloroplast biogenesis underpinning photosynthesis build-up in the wheat leaf. Genome Biol. 2021 May 11;22(1):151. doi: 10.1186/s13059-021-02366-3. PMID: 33975629; PMCID: PMC8111775.
Koester et al. (2021)Transgenic insertion of the cyanobacterial membrane protein ictB increases grain yield in Zea mays through increased photosynthesis and carbohydrate production. PLoS One. 2021 Feb 4;16(2):e0246359. doi: 10.1371/journal.pone.0246359. PMID: 33539477; PMCID: PMC7861388.
Wang et al. (2020). Post-translational coordination of chlorophyll biosynthesis and breakdown by BCMs maintains chlorophyll homeostasis during leaf development. Nat Commun. 2020; 11: 1254.
Van Gelder (2018). Medium-Chain Polyprenols Influence Chloroplast Membrane Dynamics In Solanum Lycopersicum. Plant Cell Physiol. 2018 Sep 6. doi: 10.1093/pcp/pcy157.
Fernández-San Millán et al. (2018). Physiological Performance of Transplastomic Tobacco Plants Overexpressing Aquaporin AQP1 into Chloroplast Membranes. J Exp. Bot. ery148, https://doi.org/10.1093/jxb/ery148. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Oxygenic photosynthesis poster by prof. Govindjee and Dr. Shevela
Z-scheme of photosynthetic electron transport by prof. Govindjee and Dr. Björn and Dr. Shevela - Reviews:
-
This product doesn't have any reviews.
Accessories
AS08 351 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A.thaliana, N. benthamiana, N. tabacum, P. patens, P. sativum, S. oleracea, T. salsuginea, some cross-Reactivity was observed for cyanobacteria including: Synechocystis, Synechococcus and Thermosynechococcus sp.
compartment marker of chloroplast outer envelope membrane protein marker.


