1
Anti-PsbC | CP43 protein of PSII
AS11 1787 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A. thaliana, C.reinhardtii, C. zofingiensis, E. crus-galli, H. vulgare, O. sativa, P. ginseng, P. patens, P. sativum, P. vulgaris, P. yezoensis, Synochococcus sp. PCC7002, Synechocystis sp. PCC6803, T. aestivum, Triticale, Z. mays, V. lychnitis, V. radiate
- Product Info
-
Immunogen: KLH-conjugated synthetic peptide chosen from known sequences of PsbC including Arabidopsis thaliana PsbC, UniProt: P56778, TAIR: AtCg00280
Host: Rabbit Clonality: Polyclonal Purity: Serum Format: Lyophilized Quantity: 50 µl Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Western blot (WB) Recommended dilution: 1 : 3 000 (WB) Expected | apparent MW: 45 | 43 kDa
- Reactivity
-
Confirmed reactivity: Arabidopsis thaliana, Chlamydomonas reinhardtii,Chlorella sorokiniana, Chlorella vulgaris, Chromochloris zofingiensis, Echinochloa crus-galli, Hordeum vulgare, Oryza sativa, Panax ginseng, Physcomitrium patens, Pisum sativum, Phaseolus vulgaris, Pyropia yezoensis, Synochococcus sp. PCC7002, Synechocystis sp. PCC6803, Tillandsia flabellate, Triticum aestivum, Triticale, Zea mays, Verbascum lychnitis, Vigna radiate Predicted reactivity: Asimina parviflora, Borago officinalis, Cannabis sativa, Carthamus persicus, Casimirella guaranitica , Catalpa bungei, Calatola mollis, Citron x limon, Cunninghamia lanceolata, Deeringothamnus rugelii, Gonystylus bancanus, Ipomopsis aggregata, Leretia cordata, Lobatiriccardia lobata, Myricaria germanica , Nostoc sp. PCC7120, Nannochloropsis sp., Natsiatum herpeticum, Nicotiana benthamiana, Nothapodytes montana , Nerium oleander, Ottoschulzia rhodoxylon, Oxandra lanceolata,Solanum tuberosum, Oryza sativa, Panax quinquefolius, Prosopidastrum angusticarpum, Prosopis glandulosa, Rollinia mucosa, Rosmarinus officinalis, Saxifraga rivularis, Spinacia oleracea, Zelkova serrata, Zinnia violacea, Vachellia caven, Vitis vinifera, Zosteria marina, Xerocladia viridiramis
Species of your interest not listed? Contact usNot reactive in: Diatoms - Application Examples
-
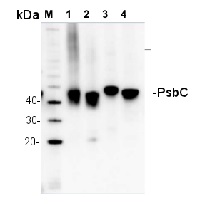
5 µg of total protein from (1) Arabidopsis thaliana leaf extracted with Protein Extration Buffer, PEB (AS08 300), (2) Hordeum vulgare leaf extracted with PEB, (3) Chlamydomonas reinhardtii total cell extracted with PEB, (4) Synechococcus sp. 7942 total cell extracted with PEB, extracted with PEB were separated on 4-12% NuPage (Invitrogen) LDS-PAGE and blotted 1h to PVDF. Blots were blocked immediately following transfer in 2% blocking reagent in 20 mM Tris, 137 mM sodium chloride pH 7.6 with 0.1% (v/v) Tween-20 (TBS-T) for 1h at room temperature with agitation. Blots were incubated in the primary antibody at a dilution of 1: 10 000 for 1h at room temperature with agitation. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 15 min and 3 times for 5 min in TBS-T at room temperature with agitation. Blots were incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, recommended secondary antibody AS09 602) diluted to 1:25 000 in 2% blocking solution for 1h at room temperature with agitation. The blots were washed as above and developed for 5 min with chemiluminescent detection reagent according the manufacturers instructions. Images of the blots were obtained using a CCD imager (FluorSMax, Bio-Rad) and Quantity One software (Bio-Rad). Exposure time was 75 seconds.
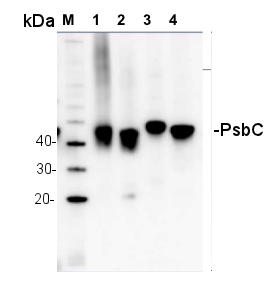
 1.5 µg of chlorophyll from thylakoids of various treatments of Echinochloa crus-galli (1-2), Zea mays (3-5), Pisum sativum (6-7), extracted with 0.4 M sorbitol, 50 mM Hepes NaOH, pH 7.8, 10 mM NaCl, 5 mM MgCl2 and 2 mM EDTA. Samples were denatured with Laemmli buffer at 75°C for 5 min and were separated on 12% SDS-PAGE and blotted 30 min to PVDF using wet transfer. Blot was blocked with 5% fatty acid free milk for 1h at room temperature (RT) with agitation. Blot was incubated in the primary antibody at a dilution of 1: 3 000 overnight at 4°C with agitation in 1% milk in TBS-T. The antibody solution was decanted and the blot was washed 4 times for 5 min in TBS-T at RT with agitation. Blot was incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, AS09 602, Agrisera ) diluted to 1:25 000 in 1 % milk in TBS-T for 1h at RT with agitation. The blot was washed 5 times for 5 min in TBS-T and 2 times for 5 min in TBS, and developed for 1 min with 1.25 mM luminol, 0.198 mM coumaric acid and 0.009% H2O2 in 0.1 M Tris- HCl, pH 8.5. Exposure time in ChemiDoc System was 240 seconds.
1.5 µg of chlorophyll from thylakoids of various treatments of Echinochloa crus-galli (1-2), Zea mays (3-5), Pisum sativum (6-7), extracted with 0.4 M sorbitol, 50 mM Hepes NaOH, pH 7.8, 10 mM NaCl, 5 mM MgCl2 and 2 mM EDTA. Samples were denatured with Laemmli buffer at 75°C for 5 min and were separated on 12% SDS-PAGE and blotted 30 min to PVDF using wet transfer. Blot was blocked with 5% fatty acid free milk for 1h at room temperature (RT) with agitation. Blot was incubated in the primary antibody at a dilution of 1: 3 000 overnight at 4°C with agitation in 1% milk in TBS-T. The antibody solution was decanted and the blot was washed 4 times for 5 min in TBS-T at RT with agitation. Blot was incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, AS09 602, Agrisera ) diluted to 1:25 000 in 1 % milk in TBS-T for 1h at RT with agitation. The blot was washed 5 times for 5 min in TBS-T and 2 times for 5 min in TBS, and developed for 1 min with 1.25 mM luminol, 0.198 mM coumaric acid and 0.009% H2O2 in 0.1 M Tris- HCl, pH 8.5. Exposure time in ChemiDoc System was 240 seconds.
Courtesy of Dr. Wiola Wasilewska, Warsaw University, PolandApplication examples: 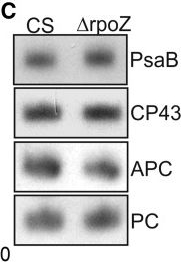
Reactant: Synechocystis
Application: Western Blotting
Pudmed ID: 24476911
Journal: Nucleic Acids Res
Figure Number: 6C
Published Date: 2014-04-01
First Author: Gunnelius, L., Hakkila, K., et al.
Impact Factor: 16.476
Open PublicationPhotosynthetic properties of ?rpoZ. (A) Light-saturated photosynthetic activity of a 1 ml of culture (OD730 = 1) of CS (white bar) and ?rpoZ (black bar) in standard conditions. Each bar represents an average of three independent biological replicates, and the error bars denote SE. (B) Fluorescence at 77 K was measured using 440-nm light that excites Chl. The data were normalized by dividing with the height of the PSI emission peak at 723 nm. (C) Total proteins were isolated, separated with SDS-PAGE and the amounts of PSI reaction center protein PsaB, PSII core protein CP43 and the phycobilisome proteins allophycocyanin (APC) and phycocyanin (PC) were measured by western blotting. The protein contents of PsaB, CP43, ACP and PC were 99 ± 6%, 97 ± 7, 103 ± 7% and 99 ± 4, respectively, in ?rpoZ of that measured in CS.
Reactant: Plant
Application: Western Blotting
Pudmed ID: 27590049
Journal: BMC Plant Biol
Figure Number: 9A
Published Date: 2016-09-02
First Author: Mazur, R., Sadowska, M., et al.
Impact Factor: 4.142
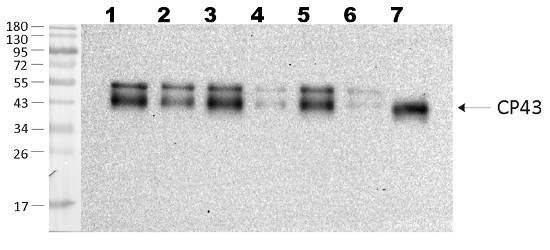
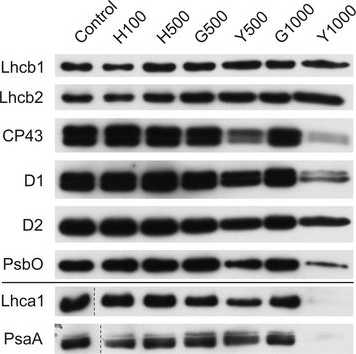
Open PublicationChanges of PSII and PSI antenna and core protein levels. Proteins from control and Tl-treated white mustard leaves were separated by SDS-PAGE followed by immunodetection with antibodies against Lhcb1, Lhcb2, Lhca1 (antenna proteins) and D1, D2, CP43, PsbO, PsaA (core proteins). Samples were loaded on the equal amount of chlorophyll (0.25 ?g). Description of samples abbreviation as given in the legend to Fig. 3
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 36460653
Journal: Nat Commun
Figure Number: 3C
Published Date: 2022-12-02
First Author: Zhang, M.
Impact Factor: 15.405
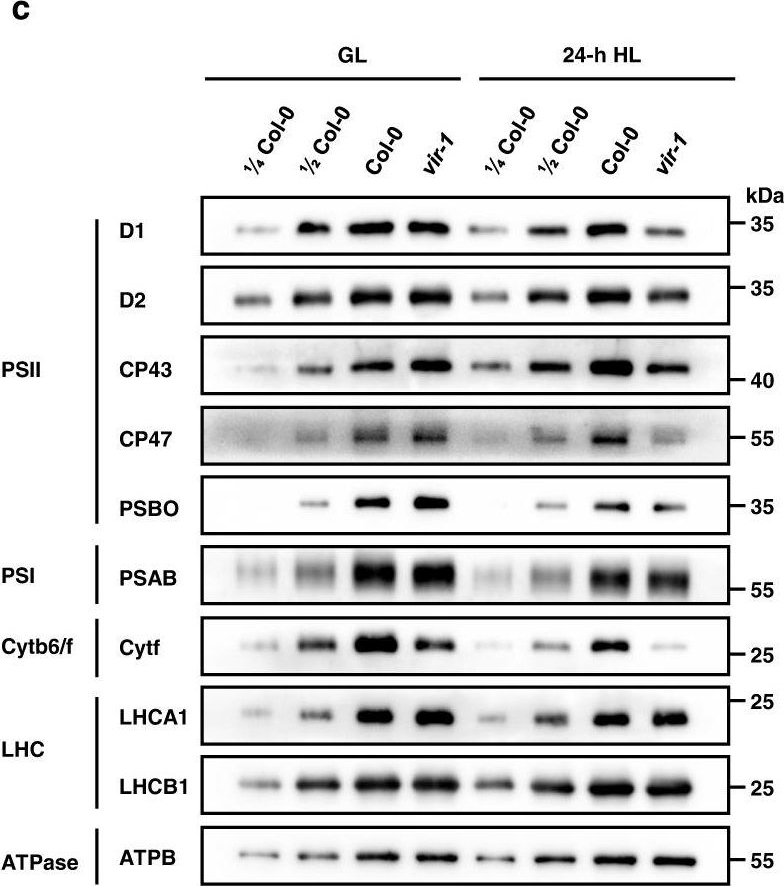
Open PublicationAnalysis of PSII complexes and subunits in Col-0 and vir-1 seedlings.a BN-PAGE and immunoblot analysis of thylakoid photosynthetic complexes. Equal amounts of thylakoid membrane (10 μg chlorophylls) from Col-0 and vir-1 seedlings under 0-h (GL) and 24-h (HL) high light treatment were solubilized with 2% n-dodecyl-β-D-maltoside (DM) and separated by BN-PAGE. The BN-PAGE gel was stained with Coomassie brilliant blue (CBB). The macromolecular protein complexes of thylakoid membranes (indicated on the left) were identified according to Jin48. For BN-PAGE immunoblot analysis, an equal amount of chlorophyll (1.5 μg) was loaded in each lane, and anti-D1, anti-D2, anti-CP43, and anti-CP47 antisera were used to probe the PSII complex. I: PSII-LHCII supercomplex; II: PSII dimer, PSI monomer; III: PSI monomer, CF1; IV: Cyt b6f, PSII core monomer; V: CP43-less PSII core monomer; VI: LHCII trimer. All experiments involved three independent biological replicates, which produced similar results. b Proteins immunodetected from (a) were quantified with Phoretix 1D software (Phoretix International, UK). Values (mean ± SE, n = 3 independent biological replicates) are given relative to protein levels of Col-0 before HL treatment. *P < 0.05; **P < 0.01, by two-sided Student’s t test. c, Analysis of thylakoid membrane protein accumulation in Col-0 and vir-1 mutants. Thylakoid membrane proteins from Col-0 and vir-1 seedlings were separated by 12% SDS-urea-PAGE and probed with antisera against specific thylakoid membrane proteins. Samples were loaded on an equal chlorophyll basis. Cyt b6f, cytochrome b6f complex; LHC, light-harvesting complex; ATPase, ATP synthase complex. Similar results were obtained from three independent biological replicates. d Proteins immunodetected from (c) were quantified with Phoretix 1D software (Phoretix International, UK). Values (means ± SE; n = 3 independent biological replicates) are given relative to protein levels of Col-0 before high light treatment. *P < 0.05; **P < 0.01, by two-sided Student’s t test.
- Additional Information
-
Additional information: Contains 0,01% ProClin Additional information (application): In C4 plants like Echinochloa crus-galli and Zea mays antibody detects 2 bands. - Background
-
Background: PsbC (CP43) acts as an antenna to the PSII core and its presence seem to be also necessary for maintaining water splitting activity. This protein is more weakly associated with the PSII reaction centre and can be removed from the isolated core.
- Product Citations
-
Selected references: Zhao et al. (2024). Psb28 protein is indispensable for stable accumulation of PSII core complexes in Arabidopsis.Plant J. 2024 May 26. doi: 10.1111/tpj.16844.
Ciesielska et al. (2024). S2P2-the chloroplast-located intramembrane protease and its impact on the stoichiometry and functioning of the photosynthetic apparatus of A. thaliana. Front Plant Sci. 2024 Mar 15:15:1372318. doi: 10.3389/fpls.2024.1372318.
Kim et al. (2024).Photoautotrophic cultivation of a Chlamydomonas reinhardtii mutant with zeaxanthin as the sole xanthophyll. Biotechnol Biofuels Bioprod. 2024 Mar 14;17(1):41. doi: 10.1186/s13068-024-02483-8.
Khaig and Eaton-Rye (2023).Lys264 of the D2 Protein Performs a Dual Role in Photosystem II Modifying Assembly and Electron Transfer through the Quinone–Iron Acceptor Complex. Biochemistry 2023, 62, 18, 2738–2750
Kafri et al. (2023). Systematic identification and characterization of genes in the regulation and biogenesis of photosynthetic machinery. Cell. 2023 Dec 7;186(25):5638-5655.e25.doi: 10.1016/j.cell.2023.11.007.
Hu C, Mascoli V, Elias E, Croce R. The photosynthetic apparatus of the CAM plant Tillandsia flabellate and its response to water deficit. J Plant Physiol. 2023 Mar;282:153945. doi: 10.1016/j.jplph.2023.153945
Cazzaniga et al. (2022). Engineering astaxanthin accumulation reduces photoinhibition and increases biomass productivity under high light in Chlamydomonas reinhardtii. Biotechnol Biofuels Bioprod. 2022 Jul 11;15(1):77. doi: 10.1186/s13068-022-02173-3. PMID: 35820961; PMCID: PMC9277849.
Xiong et al. (2022) a chloroplast nucleoid protein of bacterial origin linking chloroplast transcriptional and translational machineries, is required for proper chloroplast gene expression in Arabidopsis thaliana. Nucleic Acids Res. 2022 Jun 23;50(12):6715–34. doi: 10.1093/nar/gkac501. Epub ahead of print. PMID: 35736138; PMCID: PMC9262611.
Beckova et al. (2022). Photosystem II antenna modules CP43 and CP47 do not form a stable 'no reaction centre complex' in the cyanobacterium Synechocystis sp. PCC 6803. Photosynth Res. 2022 Jan 11. doi: 10.1007/s11120-022-00896-w. Epub ahead of print. PMID: 35015206.
Cecchin et al (2021) LPA2 protein is involved in photosystem II assembly in Chlamydomonas reinhardtii. Plant J. 2021 Jul 4. doi: 10.1111/tpj.15405. Epub ahead of print. PMID: 34218480.
Okegawa et al (2021) Maintaining the Chloroplast Redox Balance Through the PGR5-Dependent Pathway and the Trx System is Required for Light-Dependent Activation of Photosynthetic Reactions. Plant Cell Physiol. 2021 Oct 8:pcab148. doi: 10.1093/pcp/pcab148. Epub ahead of print. PMID: 34623443.
Sakuraba at al. (2020). Multilayered regulation of membrane-bound ONAC054 is essential for abscisic acid-induced leaf senescence in rice. Plant Cell. 2020 Jan 6. pii: tpc.00569.2019. doi: 10.1105/tpc.19.00569.
Trinugroho et al. (2020). Chlorophyll F Synthesis by a Super-Rogue Photosystem II Complex. Nat Plants , 6 (3), 238-244
Dong et al. (2020). Plastid ribosomal protein LPE2 is involved in photosynthesis and the response to C/N balance in Arabidopsis thaliana. J Integr Plant Biol. 2020 Jan 15. doi: 10.1111/jipb.12907.
Ma et al. (2020). Zinc toxicity alters the photosynthetic response of red alga Pyropia yezoensis to ocean acidification. Environ Sci Pollut Res Int. 2020 Jan;27(3):3202-3212. doi: 10.1007/s11356-019-06872-7.
Furukawa et al. (2019). Formation of a PSI–PSII megacomplex containing LHCSR and PsbS in the moss Physcomitrella patens. J Plant Res
Tian et al. (2019). pH dependence, kinetics and light-harvesting regulation of nonphotochemical quenching in Chlamydomonas. Proc Natl Acad Sci U S A. 2019 Apr 23;116(17):8320-8325. doi: 10.1073/pnas.
Li et al. (2019). A genome-wide algal mutant library and functional screen identifies genes required for eukaryotic photosynthesis. Nat Genet. 2019 Apr;51(4):627-635. doi: 10.1038/s41588-019-0370-6.
Rogowski et al. (2019). Photosynthesis and organization of maize mesophyll and bundle sheath thylakoids of plants grown in various light intensities. Environmental and Experimental Botany Volume 162, June 2019, Pages 72-86.
Roth et al. (2019). Regulation of Oxygenic Photosynthesis during Trophic Transitions in the Green Alga Chromochloris zofingiensis. Plant Cell. 2019 Feb 20. pii: tpc.00742.2018. doi: 10.1105/tpc.18.00742.
Schmid et al. (2018). PUMPKIN, the sole Plastid UMP Kinase, Associates with Group II Introns and Alters Their Metabolism. Plant Physiol. 2018 Nov 8. pii: pp.00687.2018. doi: 10.1104/pp.18.00687.
Mao et al. (2018). Comparison on Photosynthesis and Antioxidant Defense Systems in Wheat with Different Ploidy Levels and Octoploid Triticale. Int J Mol Sci. 2018 Oct 2;19(10). pii: E3006. doi: 10.3390/ijms19103006.
Gonzaga Heredia-Martinez et al. (2018). Chloroplast damage induced by the inhibition of fatty acid synthesis triggers autophagy in Chlamydomonas. Plant Physiol, Sept. 2018.
Liu et al. (2018). Effects of PSII Manganese-Stabilizing Protein Succinylation on Photosynthesis in the Model Cyanobacterium Synechococcus sp. PCC 7002. Plant Cell Physiol. 2018 Jul 1;59(7):1466-1482. doi: 10.1093/pcp/pcy080.
Giovanardi et al. (2018). In pea stipules a functional photosynthetic electron flow occurs despite a reduced dynamicity of LHCII association with photosystems. Biochim Biophys Acta. 2018 May 24. pii: S0005-2728(18)30129-4. doi: 10.1016/j.bbabio.2018.05.013.
Myouga et al. (2018). Stable accumulation of photosystem II requires ONE-HELIX PROTEIN1 (OHP1) of the light harvesting-like family. Plant Physiol. 2018 Feb 1. pii: pp.01782.2017. doi: 10.1104/pp.17.01782.
Kurkela et al. (2017). Acclimation to High CO2 Requires the ω Subunit of the RNA Polymerase in Synechocystis. Plant Physiol. 2017 May;174(1):172-184. doi: 10.1104/pp.16.01953. Epub 2017 Mar 28.
Chen et al. (2017). Comparison of Photosynthetic Characteristics and Antioxidant Systems in Different Wheat Strains. J Plant Growth Regul.
Gandini et al. (2017). The transporter SynPAM71 is located in the plasma membrane and thylakoids, and mediates manganese tolerance in Synechocystis PCC6803. New Phytol. 2017 Mar 20. doi: 10.1111/nph.14526.
Yang-Er Chen et al. (2017). Responses of photosystem II and antioxidative systems to high light and high temperature co-stress in wheat. J. of Exp. Botany, Volume 135, March 2017, Pages 45–55.
Yoshida et al. (2016). Hisabori T1.Two distinct redox cascades cooperatively regulate chloroplast functions and sustain plant viability. Proc Natl Acad Sci U S A. 2016 Jul 5;113(27):E3967-76. doi: 10.1073/pnas.1604101113. Epub 2016 Jun 22.
Mazur et al. (2016). Overlapping toxic effect of long term thallium exposure on white mustard (Sinapis alba L.) photosynthetic activity. BMC Plant Biol. 2016 Sep 2;16(1):191. doi: 10.1186/s12870-016-0883-4.
Kowalewska et al. (2016). Three-dimensional visualization of the internal plastid membrane network during runner bean chloroplast biogenesis. Dynamic model of the tubular-lamellar transformation. The Plant Cell March 21, 2016 tpc.01053.2015.
Chen et al. (2016). Expression of holo-proteorhodopsin in Synechocystis sp. PCC 6803. Metab Eng. 2016 Feb 8;35:83-94. doi: 10.1016/j.ymben.2016.02.001.
Liu and Last (2015). A land plant-specific thylakoid membrane protein contributes to photosystem II maintenance in Arabidopsis thaliana. Plant J. 2015 Jun;82(5):731-43. doi: 10.1111/tpj.12845. Epub 2015 Apr 29.
Yokono et al. (2015). A megacomplex composed of both photosystem reaction centres in higher plants. Nat Commun. 2015 Mar 26;6:6675. doi: 10.1038/ncomms7675.
Calderon et al. (2013). A Conserved Rubredoxin is Necessary for Photosystem II Accumulation in Diverse Oxygenic Photoautotrophs. J Biol Chem. July 30. (reference for reactivity in Chlamydomonas reinhardtii)
Sakuraba et al. (2013). The green leaf locus encodes protochlorophyllide oxidoreductase B and is essential for chlorophyll synthesis under high light conditions. Plant J.
Wientjes et al (2013). LHCII is an antenna of both photosystems after long-term acclimation. BBA, Jan 6.
Kalischuk et al. (2022) Amplification of cell signaling and disease resistance by an immunity receptor Ve1Ve2 heterocomplex in plants. Commun Biol. 2022 May 25;5(1):497. doi: 10.1038/s42003-022-03439-0. PMID: 35614138; PMCID: PMC9132969. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Oxygenic photosynthesis poster by prof. Govindjee and Dr. Shevela
Z-scheme of photosynthetic electron transport by prof. Govindjee and Dr. Björn and Dr. Shevela - Reviews:
-
Katya Georgieva | 2019-06-24Worked very well on thylakoid proteins from Haberlea rhodopensis. We used 3 ug chlorophyll per lane, 1:3000 dilution of CP43, ECL detection of WB. The antibody gave clear single band. It is possible to use lower dilution of CP43. Reference: Mihailova G, B�chel C, Dietzel L, Georgieva K (2016) Desiccation induced changes in photosynthesis related proteins of shade and sun Haberlea rhodopensis plants. Compt. Rend Bulg. Acad. Sci. 69(1), 37-44Julia Hamm | 2018-11-21antibodie shows in Synechocystis a clear bandLucja Kowalewska | 2018-06-05This antibody worked efficiently in P. coccineus in suggested dilution and blocking protocol



