1
HY1 | Heme Oxygenase 1
AS12 2636 | Clonality: Polyclonal | Host: Rabbit | Reactivity: Arabidopsis thaliana
- Data sheet
-
- Product Info
-
Immunogen: KLH-conjugated synthetic peptide derived from Arabidopsis thaliana HY1, UniProt: O48782, TAIR: At2g26670
Host: Rabbit Clonality: Polyclonal Purity: Immunogen affinity purified serum in PBS pH 7.4. Format: Lyophilized Quantity: 50 �g Reconstitution: For reconstitution add 50 �l, of sterile water Storage: Store lyophilized/reconstituted at -20�C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Western blot (WB) Recommended dilution: 1 : 1000 (WB) Expected | apparent MW: 32 kDa
- Reactivity
-
Confirmed reactivity: Arabidopsis thaliana
Predicted reactivity: Brassica juncea, Capsicum annuum, Corchorus capsularis, Morus alba, Nelumbo nucifera, Nicotiana sylvestris, Nicotiana tabacum, Noccaea caerulescens, Populus balsamifera, Populus tremula, Solanum lycopersicum
Species of your interest not listed? Contact usNot reactive in: Cyanobacteria - Application Examples
-
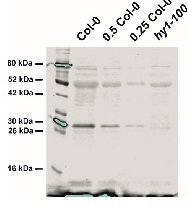
Application example 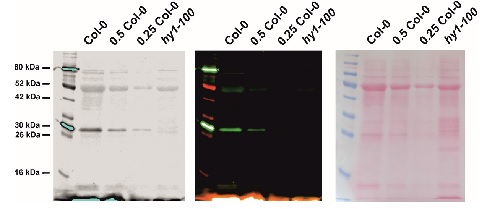
Arabidopsis thaliana wild-type (Col-0) and hy1-100 mutant seedlings were grown on ½ MS agar plates supplemented with 1% agar, without sucrose, for 2 d dark and 3 d in WLc (100 µmol m-2 s-1) at 22°C. 100 mg of cotyledon tissue was collected from 5 d old seedlings and extracted with 500 µL 2x Laemmli buffer without bromophenol blue ( 0.125 M Tris-HCl, pH 6.8; 4 % SDS; 20 % glycerol and 5 % 2-mercaptoethanol ), denatured at 99°C for 5 min using thermomixer (Eppendorf), and centrifuged for 5 min at 13,000 rpm at 4°C. Approximately 100 µg protein from 2 mg of leaf material was loaded per lane (in a 10 µL volume) with appropriate reductions for 0.5x and 0.25x, respectively. Proteins were separated on a 4 % stacking and 12 % resolving SDS-PAGE gel using standard Tris/Glycine running buffer (25 mM Tris, 192 mM glycine, 0.1 % SDS) and blotted for 1 h at 100 V to a 0.22 µm nitrocellulose membrane from Licor. A wet transfer was used, with transfer buffer containing: 20 % methanol, 25 mM Tris, 192 mM glycine. Blots were stained with Ponceau S solution (0.1 % Ponceau S in 5 % acetic acid) and washed briefly with TBS, then blocked with 5% (w/v) milk in TBS for 1 h at room temperature (RT) with agitation. Blots were incubated in the primary antibody at a dilution of 1:2000 overnight at 4°C with agitation in 5 % milk in TBS-T. The antibody solution was removed, the blot rinsed briefly, and washed six times for 5 min in TBS-T at RT with agitation. The blot was incubated in a secondary antibody protected from light (donkey anti-rabbit IgG IRDye800CW from Licor, 925-32213) diluted to 1:20000 in 5 % (w/v) milk in TBS-T for 45 min at RT with agitation. The blot was washed six times for 5 min in TBS-T at RT with agitation and three times for 2 min with TBS to remove residual Tween. For visualization, the blot was excited with the 700 nm and 800 nm channels, with scanning intensity 3 and 5, respectively, using the Odyssey infra-red blot scanner from Licor.
Courtesy of Sylwia Kacprzak and Dr. Matthew J. Terry, University of Southampton, United Kingdom - Background
-
Background: HY1 (HEME OXYGENASE 1) is a plastid heme oxygenase necessary for phytochrome chromophore biosynthesis and for coupling the expression of some nuclear genes to the functional state of the chloroplast. Alternative names: HEME OXYGENASE 1,HEME OXYGENASE 1,GENOMES UNCOUPLED 2,HO1,ARABIDOPSIS THALIANA HEME OXYGENASE 1,HY6,HY1,ATHO1,HEME OXYGENASE 6,GUN2, REVERSAL OF THE DET PHENOTYPE 4. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Agrisera Educational Posters Collection
- Reviews:
-
This product doesn't have any reviews.


