1
Anti-PsaA | PSI-A core protein of photosystem I
AS06 172 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A. thaliana, C. quitensis Kunt Bartl, C. pumilum, C. reinhardtii, C. zofingiensis, F. vesiculosus, H.vulgare, M. polymorpha, N. oceanica, N. tabacum, O. sativa, P. abies, P. sativum, P. strobus, P. vulgaris, S. oleracea, C.reinhardtii, Synechococcus PCC 7942, Synechocystis PCC 6803, Scenedesmus obliquus, microalgae N. gaditana
- Product Info
-
Immunogen: N-terminal part of recombinant PsaA protein from Chlamydomonas reinhardtii P12154
Host: Rabbit Clonality: Polyclonal Purity: Serum Format: Lyophilized Quantity: 50 µl Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Blue Native PAGE (BN-PAGE), Immunogold (IG), Western blot (WB) Recommended dilution: 1 : 20 (IG), 1 : 1000-1 : 5000 (WB) Expected | apparent MW: 82 | 55-60 kDa
- Reactivity
-
Confirmed reactivity: Arabidopsis thaliana, Begonia sp. , Bryopsis corticulans, Chlamydomonas reinhardtii, psychrophilic Chlamydomonas sp. UWO241 and Chlamydomonas sp. ICE-MDV, Chlorella sorokiniana, Chlorella vulgaris, Chromochloris zofingiensis, Colobanthus quitensis Kunt Bartl, Craterostigma pumilum, Cytisus cantabricus (Wilk.) Rchb. F., Dianthus caryophyllus, Dioxoniella giordanoi (red alga), Drosera capensis, Euonymus japonicus, Fraxinus ornus, Fucus vesiculosus, Haematococcus pluvialis, Halomicronema hongdechloris, Hieracium pilosella L., Hordeum vulgare, Lasallia hispanica, Nannochloropsis oceanica strain IMET1, Nicotiana benthamiana, Nicotiana tabacum, Oryza sativa, Pisum sativum, Marchantia polymorpha (liverwort), micro Nannochloropsis gaditana, Phaseolus vulgaris, Physcomitrium patens, Picea abies, Pinus strobus, Sinapsis alba, Spinacia oleracea, Synechococcus PCC 7942, Synechocystis PCC 6803, Syntrichia muralis (Hedw.) Raab, Scenedesmus obliquus, Tillandsia flabellate, Ulva prolifera Predicted reactivity: Algae, Bigelowiella natans, Cannabis sativa, Catalpa bungei, Citrus x limon, Cyanobacteria, Cyanidioschyzon merolae strain 10D, Galdieria sulphuraria, Lycopersicum esculentum, Panax ginseng, Picea spinulosa, Pinus thunbergii, Phaeodactylum tricornutum, Populus alba, Thermosynechococcus elongatus (strain BP-1), Triticum aestivum
Species of your interest not listed? Contact usNot reactive in: Chromera velia
- Application Examples
-
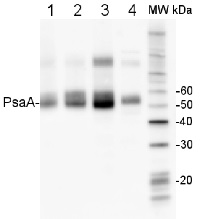
2 µg of total protein from (1) Arabidopsis thaliana leaf, (2) Hordeum vulgare leaf, (3) Chlamydomonas reinhardtii total cell, (4) Synechococcus sp. 7942 total cell all extracted with Protein Extration Buffer, PEB (AS08 300), were separated on 4-12% NuPage (Invitrogen) LDS-PAGE and blotted 1h to PVDF. Blots were blocked immediately following transfer in 2% blocking reagent in 20 mM Tris, 137 mM sodium chloride pH 7.6 with 0.1% (v/v) Tween-20 (TBS-T) for 1h at room temperature with agitation. Blots were incubated in the primary antibody at a dilution of 1: 10 000 for 1h at room temperature with agitation. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 15 min and 3 times for 5 min in TBS-T at room temperature with agitation. Blots were incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, frecommended secondary antibody AS09 602) diluted to 1:50 000 in 2% blocking solution for 1h at room temperature with agitation. The blots were washed as above and developed for 5 min with chemiluminescence detection reagent according the manufacturers instructions. Images of the blots were obtained using a CCD imager (FluorSMax, Bio-Rad) and Quantity One software (Bio-Rad). Exposure time was 10 seconds.Application examples: 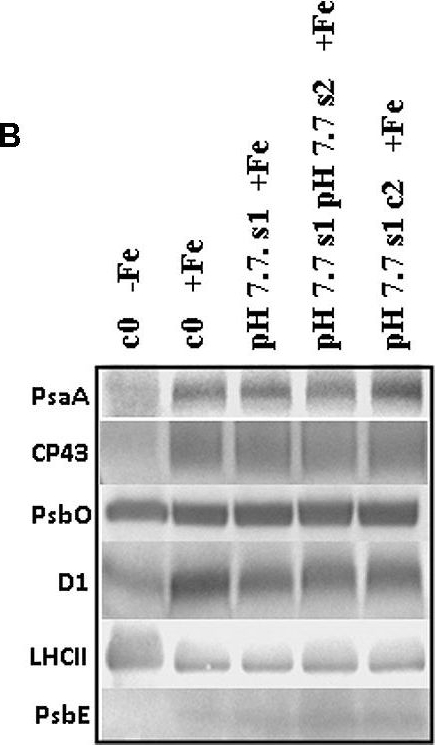
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 26442058
Journal: Front Plant Sci
Figure Number: 6B
Published Date: 2015-10-07
First Author: Murgia, I., Giacometti, S., et al.
Impact Factor: 5.435
Open PublicationO2 evolution and protein profile of photosynthetic apparatus in A. thaliana seedlings with generational exposure to Fe deficiency. (A) SHR-trap 1445 c0, pH 7.7 s1 pH 7.7 s2, pH 7.7 s1 c2 seedlings were grown for 11 days in control AIS medium and net O2 evolution (expressed as ?mol O2 evolved min-1 mg chlorophyll-1) was measured under illumination at either 100 or 800 ?E m-2 s-1. Values are mean ± SE of three biological replicas, each consisting of at least 15 seedlings. Letters represent statistical differences, according to Student’s t-test, with p < 0.05. (B) Western blot analysis with antibodies against PsaA, CP43, PsbO, D1, LHCII, and PsbE of thylakoid membranes purified from 11 days-old SHR-trap 1445 c0 seedlings germinated in -Fe AIS medium or from 11 days-old SHR-trap 1445 c0, pH 7.7 s1, pH 7.7 s1 pH 7.7 s2, pH 7.7 s1 c2 seedlings germinated in control (+Fe) AIS medium. Samples corresponding to 1 ?g chlorophyll were loaded on each lane.
Reactant: Plant
Application: Western Blotting
Pudmed ID: 27590049
Journal: BMC Plant Biol
Figure Number: 9A
Published Date: 2016-09-02
First Author: Mazur, R., Sadowska, M., et al.
Impact Factor: 4.142
Open PublicationChanges of PSII and PSI antenna and core protein levels. Proteins from control and Tl-treated white mustard leaves were separated by SDS-PAGE followed by immunodetection with antibodies against Lhcb1, Lhcb2, Lhca1 (antenna proteins) and D1, D2, CP43, PsbO, PsaA (core proteins). Samples were loaded on the equal amount of chlorophyll (0.25 ?g). Description of samples abbreviation as given in the legend to Fig. 3
Reactant: Nicotiana tabacum (Common tobacco)
Application: Western Blotting
Pudmed ID: 28180288
Journal: J Exp Bot
Figure Number: 5A
Published Date: 2017-02-01
First Author: Schöttler, M. A., Thiele, W., et al.
Impact Factor: 6.088
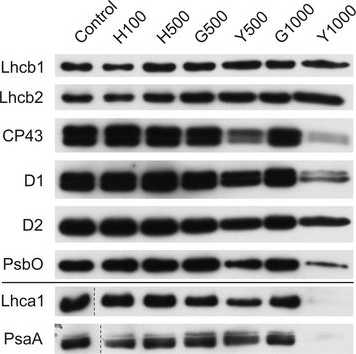
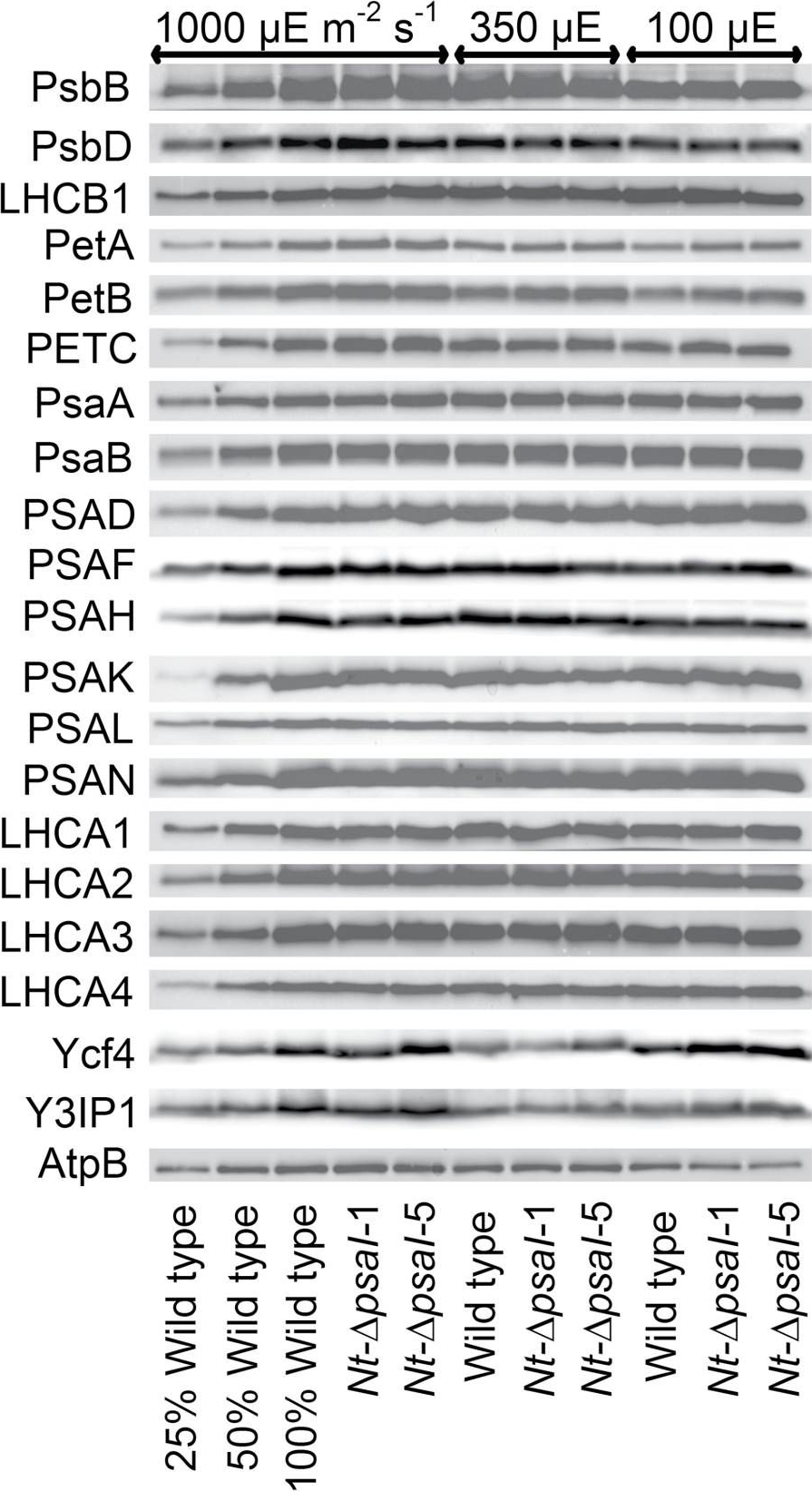
Open PublicationImmunoblot analysis of photosynthetic complex accumulation in wild-type tobacco and the two ?psaI lines grown under low, intermediate, and high-light conditions. Because the accumulation of most tested proteins was highest under high-light conditions, lanes one to three contain samples diluted to 25%, 50%, and a 100% sample of wild-type tobacco grown under high-light conditions, to allow for semi-quantitative determination of changes in protein abundance. Lanes four and five contain the two transplastomic lines grown at 1000 µE m?2 s?1. Lanes six to eight contain wild-type tobacco and the mutants grown at intermediate light intensities, and lanes nine to eleven contain samples grown at low light intensities. For PSII, the accumulation of the essential subunits PsbB (CP43) and PsbD (D2) and the LHCB1 antenna protein were determined, while for the cytochrome b6f complex, the accumulation of the essential redox-active subunits PetA (cytochrome f), PetB (cytochrome b6), and PETC (Rieske FeS protein) was tested. AtpB was probed as an essential subunit of the chloroplast ATP. For PSI, in addition to the three essential plastome-encoded subunits PsaA, PsaB, and PsaC, the accumulation of the nuclear-encoded subunits PSAD, PSAH, PSAK, PSAL, and PSAN and of the four LHCI proteins (LHCA1, LHCA2, LHCA3, LHCA4) was determined. Finally, we examined the accumulation of Ycf4, the chloroplast-encoded PSI-biogenesis factor encoded in the same operon as PsaI, and the nuclear-encoded assembly factor Y3IP1.
Reactant: Chlamydomonas reinhardtii (Green Alga)
Application: Western Blotting
Pudmed ID: 28466860
Journal: Nat Commun
Figure Number: 5A
Published Date: 2017-05-03
First Author: Fu, H. Y., Picot, D., et al.
Impact Factor: 13.783
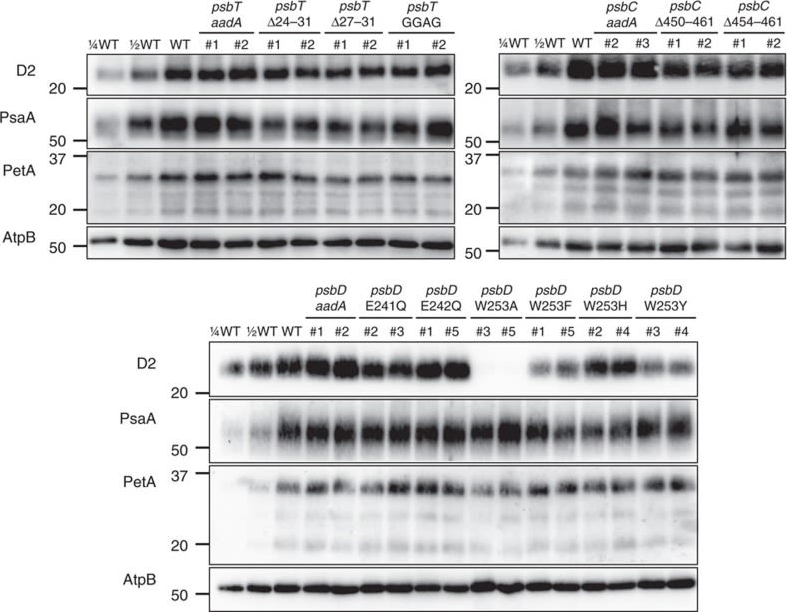
Open PublicationAccumulation of photosynthetic complexes in the mutant strains.Cells were grown in TAP medium at 25?°C under LED white light (8??mol photons m?2?s?1) and collected at the mid-log phase. Two independent lines are shown for each construct. Protein samples were loaded on an equal chlorophyll basis (0.5??g per lane), and a dilution series of WT samples is shown for semi-quantitative comparison. Antibodies against essential subunits of PSII (D2), PSI (PsaA), cytochrome b6f (PetA) and ATP synthase (AtpB) probed the accumulation of the respective photosynthetic complexes. Numbers on the left side of the blots are molecular weights in kD. See Supplementary Fig. 9 for the uncropped blot images.
Reactant: Phaeodactylum tricornutum
Application: Western Blotting
Pudmed ID: 28631733
Journal: Nat Commun
Figure Number: 2G
Published Date: 2017-06-20
First Author: Flori, S., Jouneau, P. H., et al.
Impact Factor: 13.783
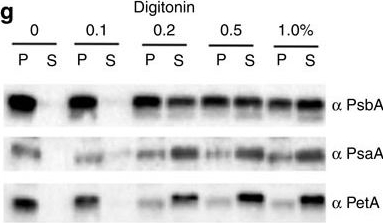
Open PublicationImmunolocalization of photosystems and of cyt b6f in the thylakoid membranes of P. tricornutum.(a–c) TEM images of P. tricornutum labelled with antibodies directed against the PsaA subunit of PSI (a), the PsbA subunit of PSII (b) and the PetA subunit of cyt b6f (c). (d) TEM micrograph of P. tricornutum thylakoid membranes showing four distinct areas: the internal membranes (‘core’: violet); the external, peripheral membranes (‘per.’: green); the pyrenoid (‘pyr.’: orange) and the envelope (‘env.’: magenta). Bars: 200?nm. (e) Principal component analysis of PSI, cyt b6f and PSII immunolocalization with the PsbA (solid squares), PsbC (open squares), PetA (cyan circle), PsaC (solid triangles) and PsaA (open triangles) antibodies. See also Supplementary Fig. 4. A total of 258 images from four independent cultures were analysed. The first two components represent more than 91% of the variance (see Supplementary Table 1, and Methods for a more detailed explanation). Green arrow: peripheral variable; violet arrow: core variable; orange arrow: pyrenoid variable; Magenta arrow: envelope variable. (f) 2D representation of the barycentre for the PSI (? PsaA+? PsaC antibodies, black square), cyt b6f (PetA, cyan circle) and PSII (? PsbA+? PsbC antibodies, red triangle) distributions. The point size along an axis is proportional to the s.d. along the corresponding component. (g) Solubilization of P. tricornutum thylakoid membranes with increasing concentrations of digitonin (0.1%, 0.2%, 0.5%, 1%). Pellet (P) and supernatant (S) were analysed by western blotting with the same anti PSI, PSII and cyt b6f antibodies as in a–c. Representative data set of an experiment replicated on three different biological samples.
Reactant: Chlamydomonas reinhardtii (Green Alga)
Application: Western Blotting
Pudmed ID: 29875782
Journal: Front Plant Sci
Figure Number: 4A
Published Date: 2018-06-08
First Author: Zhang, W., Zhong, H., et al.
Impact Factor: 5.435
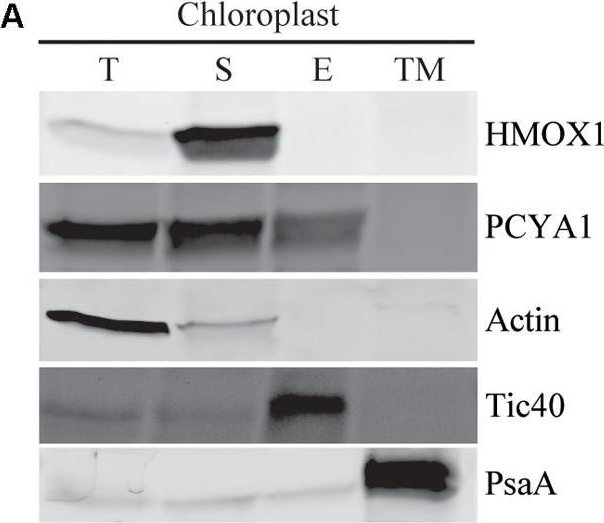
Open PublicationSubcellular distribution of CrPCYA1 in chloroplast and the export of phycocyanobilin to cytosol. (A) Biochemical fractionation of chloroplast components. Antibodies against compartmental marker proteins distributed in different chloroplast fractions were employed to distinguish the presence of CrPCYA1 via immunoblot analysis of total proteins (T), soluble fraction (S), envelope membrane (E) and thylakoid membrane (TM) from isolated chloroplast. Tic40, chloroplast envelope marker; PsaA, thylakoid membrane marker. (B) Zinc-dependent fluorescence assay. Photosensory core module (PCM) region of the prasinophyte algal DtenPHY1 purified from Chlamydomonas CC400 covalently binds phycocyanobilin (PCB). Upper panel, zinc blot; lower panel, Coomassie blue stain (CB). The two-fold serial dilutions of E. coli LMG194/pPL-PCB expressed and purified DtenPHY1 were used as positive controls. –PCB, without addition of PCB; +PCB, assembly with PCB in vitro.
Reactant: Chlamydomonas reinhardtii (Green Alga)
Application: Western Blotting
Pudmed ID: 33280077
Journal: Photosynth Res
Figure Number: 3A
Published Date: 2021-01-01
First Author: Virtanen, O., Khorobrykh, S., et al.
Impact Factor: 3.116
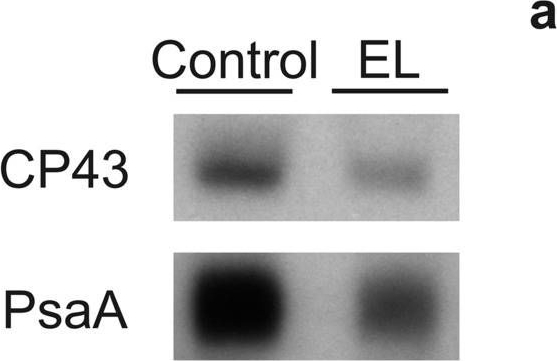
Open PublicationDetection of CP43 and PsaA proteins on a film (a) and quantification of these proteins (b) from control (white bars) and EL (black bars) cells. Western blotting was done from extracted total soluble proteins, and 1 µg (CP43) or 2 µg (PsaA) of total proteins were loaded to SDS-PAGE per well. Binding of the primary and secondary antibodies was visualized via luminescence emitted by alkaline phosphatase. The signals were normalized to the average of signals originating from control samples of the respective western blot. Each bar represents an average of three biological replicates and the error bars show SD
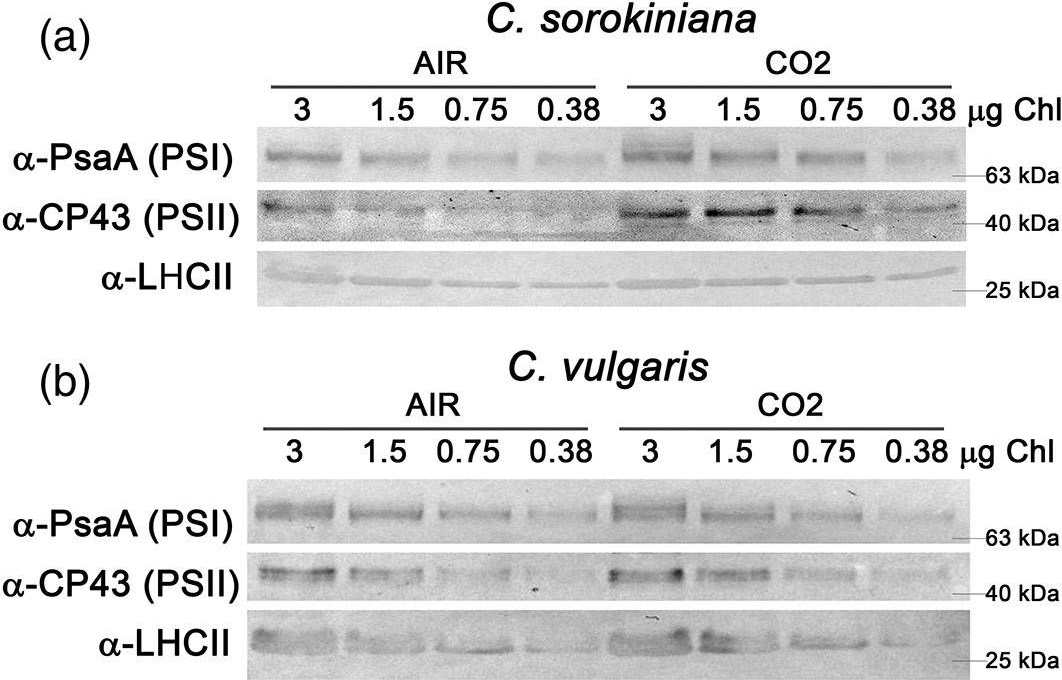
Reactant: Chlorella
Application: Western Blotting
Pudmed ID: 33931891
Journal: Plant Cell Environ
Figure Number: 5A
Published Date: 2021-09-01
First Author: Cecchin, M., Paloschi, M., et al.
Impact Factor: 6.599
Open PublicationAnalysis of PSI, PSII and LHCII content by immunoblots, P700 activity and functional PSII antenna size. (a, b) Immunoblot analysis of PSI (??PsaA antibody), PSII (??CP43 antibody) and LHCII (??LHCII antibody). Loading was performed on a chlorophyll basis: total ?g of chlorophylls loaded in each lane is reported on the top of Panel a and b. (c, d) PSI/PSII (c) and LHCII/PSII (d) ratios calculated by densitometry of immunoblot signals for C. sorokiniana (panel a, grey colour) and C. vulgaris (panel b, red colour) in AIR (full colour) or CO2 (dash colour) condition. (e) Maximal P700 oxidation on a chlorophyll basis in C. sorokiniana (left, grey colour) and C. vulgaris (right, red colour) in AIR (full colour) or CO2 (dash colour) normalized to AIR condition. (f) Functional antenna size of the photosystem II (1/?2/3) normalized to AIR condition in C. sorokiniana (grey colour) and C. vulgaris (red colour). Data are means of three biological replicates with standard deviation shown. Significant different values in CO2 versus AIR are indicated by ** (p?<?.01) and by * (p?<?.05) [Colour figure can be viewed at wileyonlinelibrary.com]
- Additional Information
-
Additional information: PsaA is a hydrophobic protein and we recommend to use PVDF membrane for transfer to assure best results.
This product can be sold containing ProClin if requested.Additional information (application): Immunogold localization has been done in leaf material of Arabidopsis thaliana.
- Background
-
Background: PsaA is a core protein of photosystem I. In plants and cyanobacteria, the primary step in oxygenic photosynthesis, the light induced charge separation, is driven bytwo large membrane intrinsic protein complexes, the photosystems I and II. Synonym: Photosystem I P700 chlorophyll a apoprotein A1.
- Product Citations
-
Selected references: Penzler et al. (2024). A pgr5 suppressor screen uncovers two distinct suppression mechanisms and links cytochrome b6f complex stability to PGR5. Plant Cell. 2024 Mar 27:koae098. doi: 10.1093/plcell/koae098.
Mu et al. (2024). Plastid HSP90C C-terminal extension region plays a regulatory role in chaperone activity and client binding.Plant J. 2024 Jul 5.doi: 10.1111/tpj.16917.
Zhao et al. (2024). Psb28 protein is indispensable for stable accumulation of PSII core complexes in Arabidopsis.Plant J. 2024 May 26. doi: 10.1111/tpj.16844.
Kim et al. (2024). Photoautotrophic cultivation of a Chlamydomonas reinhardtii mutant with zeaxanthin as the sole xanthophyll. Biotechnol Biofuels Bioprod. 2024 Mar 14;17(1):41. doi: 10.1186/s13068-024-02483-8.
Khaig and Eaton-Rye (2023).Lys264 of the D2 Protein Performs a Dual Role in Photosystem II Modifying Assembly and Electron Transfer through the Quinone–Iron Acceptor Complex. Biochemistry 2023, 62, 18, 2738–2750
Okegawa et al. (2023). x- and y-type thioredoxins maintain redox homeostasis on photosystem I acceptor side under fluctuating light. Plant Physiol. 2023 Nov 22;193(4):2498-2512.doi: 10.1093/plphys/kiad466.
Sulli et al. (2023). Generation and physiological characterization of genome‑edited Nicotiana benthamiana plants containing zeaxanthin as the only leaf xanthophyll. Planta . 2023 Oct 5;258(5):93. doi: 10.1007/s00425-023-04248-3.
Jiang et al. (2023). Toxic effects of lanthanum (III) on photosynthetic performance of rice seedlings: Combined chlorophyll fluorescence, chloroplast structure and thylakoid membrane protein assessment. Ecotoxicol Environ Saf. 2023 Nov 15:267:115627.doi: 10.1016/j.ecoenv.2023.115627.
Kafri et al. (2023). Systematic identification and characterization of genes in the regulation and biogenesis of photosynthetic machinery. Cell. 2023 Dec 7;186(25):5638-5655.e25.doi: 10.1016/j.cell.2023.11.007.
Nagy et al. (2023). Photoautotrophic and sustained H2 production by the pgr5 mutant of Chlamydomonas reinhardtii in simulated daily light conditions. International Journal of Hydrogen Energy Volume 53, 31 January 2024, Pages 760-769.
Hu et al. (2023). Drought affects both photosystems in Arabidopsis thaliana. New Phytol. 2023 Oct;240(2):663-675. doi: 10.1111/nph.19171. Epub 2023 Aug 2.
Natale et al. (2023) Structure and function of bark and wood chloroplasts in a drought-tolerant tree (Fraxinus ornus L.)
Hu C, Mascoli V, Elias E, Croce R. The photosynthetic apparatus of the CAM plant Tillandsia flabellate and its response to water deficit. J Plant Physiol. 2023 Mar;282:153945. doi: 10.1016/j.jplph.2023.153945
Chen et al. (2023) Variation of CRS2 Affected the Establishment of Photosynthetic System in Rice. Int J Mol Sci. 2023 Mar 18;24(6):5796. doi: 10.3390/ijms24065796
Kondo et al. (2023) Changes in intracellular energetic and metabolite states due to increased galactolipid levels in Synechococcus elongatus PCC 7942 [published correction appears in Sci Rep. 2023 Feb 15;13(1):2726]. Sci Rep. 2023;13(1):259. Published 2023 Jan 5. doi:10.1038/s41598-022-26760-4
Vidal-Meireles, et al. (2023)The lifetime of the oxygen-evolving complex subunit PSBO depends on light intensity and carbon availability in Chlamydomonas. Plant Cell Environ. 2023;46(2):422-439. doi:10.1111/pce.14482
von Bismarck, et al (2023). Light acclimation interacts with thylakoid ion transport to govern the dynamics of photosynthesis in Arabidopsis. New Phytol. 2023;237(1):160-176. doi:10.1111/nph.18537
Guardini et al. (2022). Loss of a single chlorophyll in CP29 triggers re-organization of the Photosystem II supramolecular assembly. Biochim Biophys Acta Bioenerg. 2022 Jun 1;1863(5):148555. doi: 10.1016/j.bbabio.2022.148555. Epub 2022 Apr 2. PMID: 35378087.
Cazzaniga et al. (2022). Engineering astaxanthin accumulation reduces photoinhibition and increases biomass productivity under high light in Chlamydomonas reinhardtii. Biotechnol Biofuels Bioprod. 2022 Jul 11;15(1):77. doi: 10.1186/s13068-022-02173-3. PMID: 35820961; PMCID: PMC9277849.
Xiong et al. (2022) a chloroplast nucleoid protein of bacterial origin linking chloroplast transcriptional and translational machineries, is required for proper chloroplast gene expression in Arabidopsis thaliana. Nucleic Acids Res. 2022 Jun 23;50(12):6715–34. doi: 10.1093/nar/gkac501. Epub ahead of print. PMID: 35736138; PMCID: PMC9262611.
Neusius et al. (2022) Lysine acetylation regulates moonlighting activity of the E2 subunit of the chloroplast pyruvate dehydrogenase complex in Chlamydomonas. Plant J. 2022 Sep;111(6):1780-1800. doi: 10.1111/tpj.15924. Epub 2022 Aug 8. PMID: 35899410.
Penzler et al. (2022) Commonalities and specialties in photosynthetic functions of PROTON GRADIENT REGULATION5 variants in Arabidopsis. Plant Physiol. 2022;190(3):1866-1882. doi:10.1093/plphys/kiac364
Urban, Rogowski & Romanowska (2022), Crucial role of the PTOX and CET pathways in optimizing ATP synthesis in mesophyll chloroplasts of C3 and C4 plants, Environmental and Experimental Botany, Volume 202, October 2022, 105024, https://doi.org/10.1016/j.envexpbot.2022.105029
Ivanov et al. (2022) The decreased PG content of pgp1 inhibits PSI photochemistry and limits reaction center and light-harvesting polypeptide accumulation in response to cold acclimation. Planta 255, 36 (2022). https://doi.org/10.1007/s00425-022-03819-0
Lim et al (2022). Arabidopsis guard cell chloroplasts import cytosolic ATP for starch turnover and stomatal opening. Nat Commun. 2022 Feb 3;13(1):652. doi: 10.1038/s41467-022-28263-2. PMID: 35115512; PMCID: PMC8814037.
Lim et al (2022) Arabidopsis guard cell chloroplasts import cytosolic ATP for starch turnover and stomatal opening. Nat Commun. 2022 Feb 3;13(1):652. doi: 10.1038/s41467-022-28263-2. PMID: 35115512; PMCID: PMC8814037.
Spaniol et al. (2022) Complexome profiling on the Chlamydomonas lpa2 mutant reveals insights into PSII biogenesis and new PSII associated proteins, Journal of Experimental Botany, Volume 73, Issue 1, 5 January 2022, Pages 245–262
Rogowski et al. (2021) Light as a substrate: migration of LHCII antennas in extended Michaelis-Menten model for PSI kinetics. J Photochem Photobiol B. 2021 Dec;225:112336. doi: 10.1016/j.jphotobiol.2021.112336. Epub 2021 Oct 19. PMID: 34736069.
Wada et al. (2021) Identification of a Novel Mutation Exacerbated the PSI Photoinhibition in pgr5/pgrl1 Mutants; Caution for Overestimation of the Phenotypes in Arabidopsis pgr5-1 Mutant. Cells. 2021 Oct 26;10(11):2884. doi: 10.3390/cells10112884. PMID: 34831107; PMCID: PMC8616342.
von Bismarck et al. (2021) Light acclimation interacts with thylakoid ion transport to govern the dynamics of photosynthesis. Research Square; 2021. DOI: 10.21203/rs.3.rs-948381/v1.
Fattore et al. (2021). Acclimation of photosynthetic apparatus in the mesophilic red alga Dixoniella giordanoi. Physiol Plant. 2021 Nov;173(3):805-817. doi: 10.1111/ppl.13489. Epub 2021 Jul 5. PMID: 34171145; PMCID: PMC8596783.
Guardini et al. (2021). High Carotenoid Mutants of Chlorella vulgaris Show Enhanced Biomass Yield under High Irradiance. Plants 10, no. 5: 911.
Lu et al. (2021). Role of an ancient light-harvesting protein of PSI in light absorption and photoprotection. Nat Commun. 2021 Jan 29;12(1):679. doi: 10.1038/s41467-021-20967-1. PMID: 33514722; PMCID: PMC7846763. (blue-native PAGE)
Kobayashi et al. (2020). Relationship Between Glycerolipidsand Photosynthetic Components During Recovery of Thylakoid Membranes From NitrogenStarvation-Induced Attenuation in Synechocystis sp. PCC 6803. Front Plant Sci. 2020 Apr 15;11:432. doi: 10.3389/fpls.2020.00432. eCollection 2020.
Their et al. (2020). VIPP2 interacts with VIPP1 and HSP22E/F at chloroplast membranes and modulates a retrograde signal for HSP22E/F gene expression. Plant Cell Environ. 2020 Jan 29. doi: 10.1111/pce.13732.
Jokel et al. (2020). Elimination of the flavodiiron electron sink facilitates long-term H2 photoproduction in green algae. Biotechnol Biofuels. 2019 Dec 5;12:280. doi: 10.1186/s13068-019-1618-1.
Liu et al. (2020). Acid treatment combined with high light leads to increased removal efficiency of Ulva prolifera. Algal Research,Volume 45, January 2020, 101745
Zhong et al. (2019). Slower development of PSI activity limits photosynthesis during Euonymus japonicus leaf development. Plant Physiol Biochem. 2019 Mar;136:13-21. doi: 10.1016/j.plaphy.2019.01.004.
Roth et al. (2019). Regulation of Oxygenic Photosynthesis during Trophic Transitions in the Green Alga Chromochloris zofingiensis. Plant Cell. 2019 Feb 20. pii: tpc.00742.2018. doi: 10.1105/tpc.18.00742.
Bastow et al. (2018). Vacuolar Iron Stores Gated by NRAMP3 and NRAMP4 Are the Primary Source of Iron in Germinating Seeds. Plant Physiol. 2018 Jul;177(3):1267-1276. doi: 10.1104/pp.18.00478.
Kato et al. (2018). Stepwise evolution of supercomplex formation with photosystem I is required for stabilization of chloroplast NADH dehydrogenase-like complex: Lhca5-dependent supercomplex formation in Physcomitrella patens. Plant J. 2018 Sep 3. doi: 10.1111/tpj.14080.
Zhang et al. (2018). VIRESCENT-ALBINO LEAF 1 regulates leaf colour development and cell division in rice. J Exp Bot. 2018 Aug 8. doi: 10.1093/jxb/ery250.
Giovanardi et al. (2018). In pea stipules a functional photosynthetic electron flow occurs despite a reduced dynamicity of LHCII association with photosystems. Biochim Biophys Acta. 2018 May 24. pii: S0005-2728(18)30129-4. doi: 10.1016/j.bbabio.2018.05.013.
Pao et al. (2018). Lamelloplasts and minichloroplasts in Begoniaceae: iridescence and photosynthetic functioning. J Plant Res. 2018 Mar 2. doi: 10.1007/s10265-018-1020-2. (ImmunoGold)
He at al. (2018). FRUCTOKINASE-LIKE PROTEIN 1 interacts with TRXz to regulate chloroplast development in rice. J Integr Plant Biol. 2018 Feb;60(2):94-111. doi: 10.1111/jipb.12631.
Myouga et al. (2018). Stable accumulation of photosystem II requires ONE-HELIX PROTEIN1 (OHP1) of the light harvesting-like family. Plant Physiol. 2018 Feb 1. pii: pp.01782.2017. doi: 10.1104/pp.17.01782.
Muneer et al. (2018). Proteomic Analysis Reveals the Dynamic Role of Silicon in Alleviation of Hyperhydricity in Carnation Grown In Vitro. Int. J. Mol. Sci. 2018, 19(1), 50; doi:10.3390/ijms19010050.
Schottler et al. (2017). The plastid-encoded PsaI subunit stabilizes photosystem I during leaf senescence in tobacco. J Exp Bot. 2017 Feb 1;68(5):1137-1155. doi: 10.1093/jxb/erx009.
Fu et al. (2017). Redesigning the QA binding site of Photosystem II allows reduction of exogenous quinones. Nat Commun. 2017 May 3;8:15274. doi: 10.1038/ncomms15274. (Chlamydomonas reinhardtii)
Sakuraba et al. (2017). Rice Phytochrome-Interacting Factor-Like1 (OsPIL1) is involved in the promotion of chlorophyll biosynthesis through feed-forward regulatory loops. Journal of Experimental Botany doi:10.1093/jxb/erx231.
Gandini et al. (2017). The transporter SynPAM71 is located in the plasma membrane and thylakoids, and mediates manganese tolerance in Synechocystis PCC6803. New Phytol. 2017 Mar 20. doi: 10.1111/nph.14526. (BN-PAGE)
Miguez et al. (2017). Diversity of winter photoinhibitory responses: A case study in co-occurring lichens, mosses, herbs and woody plants from subalpine environments. Physiol Plant. 2017 Feb 14. doi: 10.1111/ppl.12551.
Mazur et al. (2016). Overlapping toxic effect of long term thallium exposure on white mustard (Sinapis alba L.) photosynthetic activity. BMC Plant Biol. 2016 Sep 2;16(1):191. doi: 10.1186/s12870-016-0883-4.
Yoshida et al. (2016). Hisabori T1.Two distinct redox cascades cooperatively regulate chloroplast functions and sustain plant viability. Proc Natl Acad Sci U S A. 2016 Jul 5;113(27):E3967-76. doi: 10.1073/pnas.1604101113. Epub 2016 Jun 22.
Gerotto et al. (2016). Flavodiiron proteins act as safety valve for electrons in Physcomitrella patens. PNAS DOI 10.1073.
Pavlovic et al. (2016). A carnivorous sundew plant prefers protein over chitin as a source of nitrogen from its traps. Plant Physiol Biochem. 2016 Mar 5;104:11-16. doi: 10.1016/j.plaphy.2016.03.008
Pavlovic et al. (2016). Light-induced gradual activation of photosystem II in dark-grown Norway spruce seedlings. Biochim Biophys Acta. 2016 Feb 18. pii: S0005-2728(16)30028-7. doi: 10.1016/j.bbabio.2016.02.009.
Jin et al. (2023) Dual roles for CND1 in maintenance of nuclear and chloroplast genome stability in plants. Cell Rep. 2023 Mar 28;42(3):112268. doi: 10.1016/j.celrep.2023.112268. Epub 2023 Mar 17. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Oxygenic photosynthesis poster by prof. Govindjee and Dr. Shevela
Z-scheme of photosynthetic electron transport by prof. Govindjee and Dr. Björn and Dr. Shevela - Reviews:
-
Soujanya Kuntam | 2021-11-09We have used this product for many years and it has worked well with no problems in C. reinhardtiiZizhen Liang | 2019-06-27I used this antibody for many years, I really appreciate its high specificity.Veronika Z | 2014-09-29WB was performed on N. tabacum leaf in 10% ?SDS-PAGE. Dilution antibody 1:10 000. Protein was observed in area 55 kDa.Jun Liu | 2014-01-14Analysis perfomed on Arabidopsis total leaf extract, 12% SDS gel, 15 ug protein per lane, AB dilution 1:20 000, the product observed at around 60 kDa.
Accessories
AS09 461 | Clonality: Polyclonal | Host: Rabbit | Reactivity: Plants (monocots and dicots, conifers), moss: Physcomitrella patens, Chlamydomonas reinhardtii, Triticum aestivum, cyanobacteria
compartment marker of thylakoid membrane


