1
Anti-Fd-GOGAT | Ferredoxin-dependent Glutamate synthase
- Product Info
-
Immunogen: Purified full-length, tag cleaved, recombinant Zea mays GOGAT, UniProt: P23225
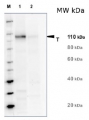
Host: Rabbit Clonality: Polyclonal Purity: Total IgG. Protein A purified in PBS, 50% glycerol. Filter sterilized. Format: Liquid at 2 mg/ml. Quantity: 100 µg Storage: Store at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: ELISA (ELISA), Western blot (WB) Recommended dilution: 1: 2000 - 1: 5000 (WB) Expected | apparent MW: 175 kDa (Zea mays), 168 kDa (Arabidopsis thaliana) - Reactivity
-
Confirmed reactivity: Arabidopsis thaliana, Spinacia oleracea, Synechocystis sp. PCC6803, Zea mays Predicted reactivity: Arthrospira platensis
Species of your interest not listed? Contact usNot reactive in: No confirmed exceptions from predicted reactivity are currently known - Application Examples
-
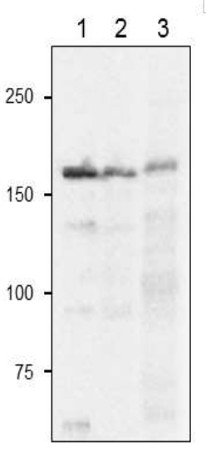
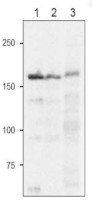
Recombinant FdGOGAT from Zea mays (1), 10 µg of Arabidopsis thaliana total leaf extract (2), 10 µg of Zea mays total leaf extract (3),were freshly extracted with 2x SDS-sample buffer (+ 2ME) for SDS-PAGE and denatured with 4X SDS buffer at 95°C for 5 min. Samples were separated on 10% SDS-PAGE and blotted 1h to PVDF membrane. Blot was blocked with 3 % skim milk/TBS-T, 1h/RT with agitation. Blot was incubated in the primary antibody at a dilution of 1: 2500 in TBS-T for 1h/RT. The antibody solution was decanted and the blot was washed 4 times for 10 min in TBS-T at RT with agitation. Blot was incubated in matching secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated) diluted to 1:10 000 in for 1h/RT with agitation. The blot was washed as above and developed with a chemiluminescent detection reagent, following manufacture's recommendation.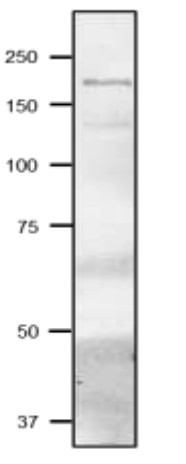
Total cell extract from Synechocystis PCC6803 freshly extracted with 2x SDS-sample buffer (+ 2ME) for SDS-PAGE and denatured with 4X SDS buffer at 95°C for 5 min. Samples were separated on 10% SDS-PAGE and blotted 1h to PVDF membrane. Blot was blocked with 3 % skim milk/TBS-T, 1h/RT with agitation. Blot was incubated in the primary antibody at a dilution of 1: 2000 in TBS-T for 1h/RT. The antibody solution was decanted and the blot was washed 4 times for 10 min in TBS-T at RT with agitation. Blot was incubated in matching secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated) diluted to 1:10 000 in for 1h/RT with agitation. The blot was washed as above and developed with a chemiluminescent detection reagent, following manufacture's recommendation. - Background
-
Background: Glutamine oxoglutarate aminotransferase (GOGAT) is an enzyme involved in synthesis of glutamate from glutamine and alpha-ketoglutarate. GOGAT has two forms in plants: ferredoxin-dependent GOGAT (Fd-GOGAT) and NADH-dependent GOGAT (NADH-GOGAT). 95% of GOGAT found in plants is the Fd-GOGAT type. Fd-GOGAT is encoded by two genes, glu1 and glu2 in Arabidopsis. Fd-GOGAT (both forms) is highly conserved among plants, red algae, and cyanobacteria. Ferredoxin-dependent glutamate synthase, chloroplastic (Fd-GOGAT) is involved in glutamate biosynthesis in leaf. This protein required for the reassimilation of ammonium ions generated during photorespiration. Gene name is GlsF. - Product Citations
-
Selected references: Ariga and Hase (2014). Multiple complexes of nitrogen assimilatory enzymes in spinach chloroplasts: possible mechanisms for the regulation of enzyme function. PLoS One. Oct 1;9(10):e108965. doi: 10.1371/journal.pone.0108965.
Sakaibara et al. (1991). Molecular cloning and characterization of complementary DNA encoding for ferredoxin-dependent glutamate synthase in maize leaf. J Biol Chem. Feb 5;266(4):2028-35. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Agrisera Educational Posters Collection - Reviews:
-
This product doesn't have any reviews.
Accessories

AS07 242 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A. thaliana, G. max, N. tabacum, O. sativa, Populus sp., S. lycopersicum, S. elongatus, S. oleracea , Z. mays
Benefits of using this antibody