1

Anti-Lhcb1 | LHCII type I chlorophyll a/b-binding protein
AS01 004 | Clonality: Polyclonal | Host: Rabbit | Reactivity: Photosynthetic eukaryotes including A. thaliana, A. hypogaea, B. napus, C.sinensis, C. reticulata, C. vulgaris, C. quitensis Kunt Bartl, C. pumilum, H. vulgare, L. esculentum (Solanum lycopersicon), M. crystallinum, N. tabacum, O. sativa, P. sativum, P. vulgaris, R. discolor, S. alaba, S. vulgaris, S. lycopersicum, S. oleracea, T. aestivum, Z. mays
Replaced by AS09 522 (A.thaliana)
- Data sheet
-
- Product Info
-
Immunogen: BSA-conjugated, 11 amino acidslong synthetic peptide derived from a highly conserved sequence of Lhb1 proteins from angiosperms (monocots and dicots) and gymnosperms, including Arabidopsis thaliana Lhcb1.1 UniProt: P0CJ48-1, TAIR: AT1G29920, Lhcb1.2 UniProt: Q8VZ87-1, TAIR: AT1G29910, Lhcb1.3 (most expressed) UniProt: P04778-1, TAIR: AT1G2993, Lhcb1.4 UniProt: Q39142-1 , TAIR: AT2G34430, Lhcb1.5 UniProt: Q39141-1, TAIR: At2g34420
Host: Rabbit Clonality: Polyclonal Purity: Immunogen affinity purified serum in PBS pH 7.4. Format: Lyophilized Quantity: 50 µg Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Western blot (WB) Recommended dilution: 1 : 2000 (WB) Expected | apparent MW: 28 | 25 kDa for Arabidopsis thaliana
- Reactivity
-
Confirmed reactivity: Arabidopsis thaliana, Arachis hypogaea, Brassica napus, Camelina sinensis, Citrus retuculata, Chlorella vulgaris, Colobanthus quitensis Kunt Bartl, Craterostigma pumilum, Hordeum vulgare, Lycopersicon esculentum (Solanum lycopersicon), Mesembryanthemum crystallinum, Nicotiana benthamiana, Nicotiana tabacum, Oryza sativa, Pisum sativum, Phaseolus vulgaris, Rhoeo discolor, Silene vulgaris, Sinapsis alba, Solanum lycopersicum, Spinacia oleracea, Tillandsia flabellate, Triticum aestivum, Triticale, Zea mays Predicted reactivity: Aegilops tauschii, Catalpa bungei, Cucumis sativus, Brachypodium distachyon, Lotus japonicus, Hordeum vulgare, Musa acuminata, Physcomitrium patens, Saccharum sp, Solanum tuberosum, Zosteria marina, Vitis vinifera
Species of your interest not listed? Contact usNot reactive in: Picea abies - Application Examples
-
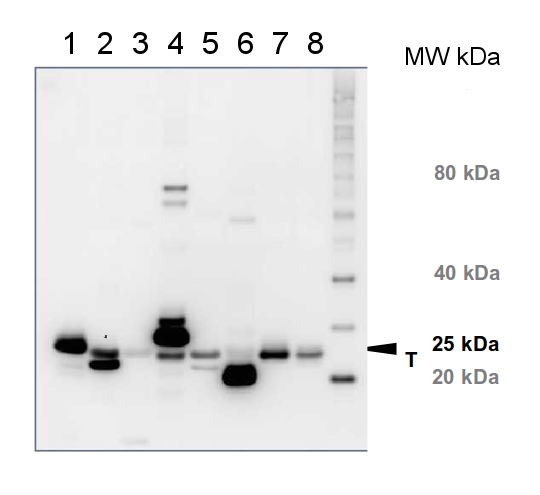
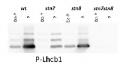
10 µg of total protein from (1) Arabidopsis thaliana leaf, (2) Hordeum vulgare leaf, (3) Zea mays leaf, (4) Chlamydomonas reinhardtii total cell, (5) Spinacia oleracea total leaf, (6) Physcomitrella patens, (7) Solanum tuberosum total leaf, (8) Solanum esculentum total leaf, all extracted with Protein Extraction Buffer PEB (AS08 300) were separated on 4-12% NuPage (Invitrogen) LDS-PAGE and blotted 1h to PVDF. Blots were blocked immediately following transfer in 2-2.5 % blocking reagent in 20 mM Tris, 137 mM sodium chloride pH 7.6 with 0.1% (v/v) Tween-20 (TBS-T) for 1h at room temperature with agitation. Blots were incubated in the primary antibody at a dilution of 1: 10 000 for 1h at room temperature with agitation. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 15 min and 3 times for 5 min in TBS-T at room temperature with agitation. Blots were incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, recommended secondary antibody AS09 602) diluted to 1:25 000 in TBS-T for 1h at room temperature with agitation. The blots were washed as above and developed for 5 min with chemiluminescent detection reagent according the manufacturers instructions. Images of the blots were obtained using a CCD imager (FluorSMax, Bio-Rad) and Quantity One software (Bio-Rad). Exposure time was 2 minutes.Application examples: 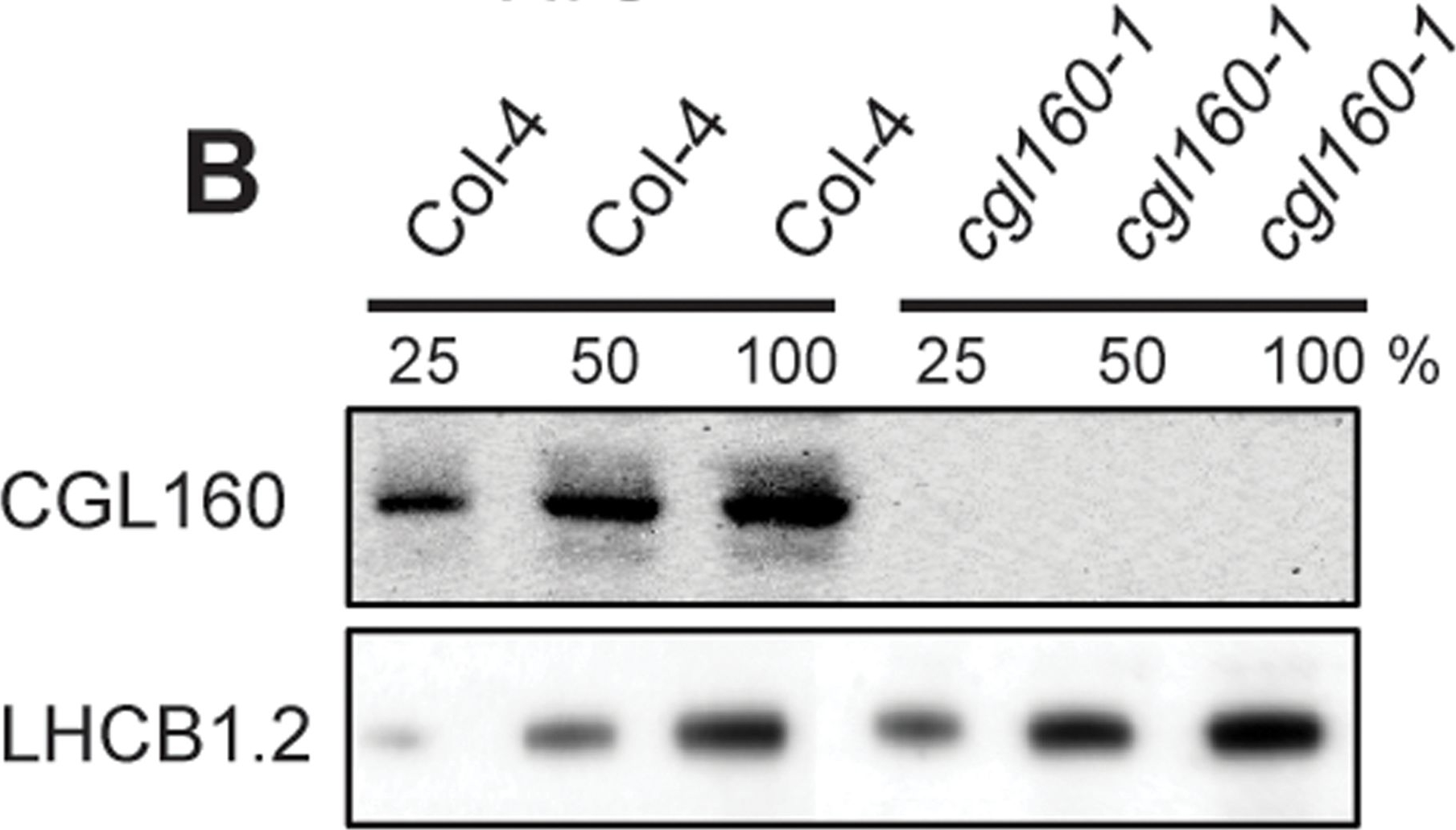
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 25835989
Journal: PLoS One
Figure Number: 2B
Published Date: 2015-04-04
First Author: Fristedt, R., Martins, N. F., et al.
Impact Factor: 2.942
Open PublicationIsolation and characterization of Arabidopsis cgl160 mutants.A. DNA insertion sites in CGL160 gene of Arabidopsis. DNA insertion sites (black triangles) are shown in relation to the CGL160 gene structure. The two cgl160 alleles analyzed in this study are denoted as cgl160-1 and cgl160-2. The CGL160 coding region is indicated by the translational start (ATG). The CGL160 genomic locus contains nine exons but only the first four are shown in the fig (grey boxes), shown are also the first four introns (black thin connecting lines). Before ATG is the promoter region in light gray. The region used for CGL160 specific antibody is shown as antigen. B. Characterization of CGL160 amount in Arabidopsis cgl160-1 mutant from isolated chloroplasts. The CGL160 antibody was used for immunoblotting and 10 ?g protein was loaded in each lane. The LHCB1 antibody was used as a loading control. C. Characterization of CGL160 amount in Arabidopsis cgl160-2 mutant from isolated chloroplasts. The CGL160 antibody was used for immunoblotting and 10 ?g protein was loaded in each lane. The LHCB1 antibody was used as a loading control.
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 25835989
Journal: PLoS One
Figure Number: 2C
Published Date: 2015-04-04
First Author: Fristedt, R., Martins, N. F., et al.
Impact Factor: 2.942
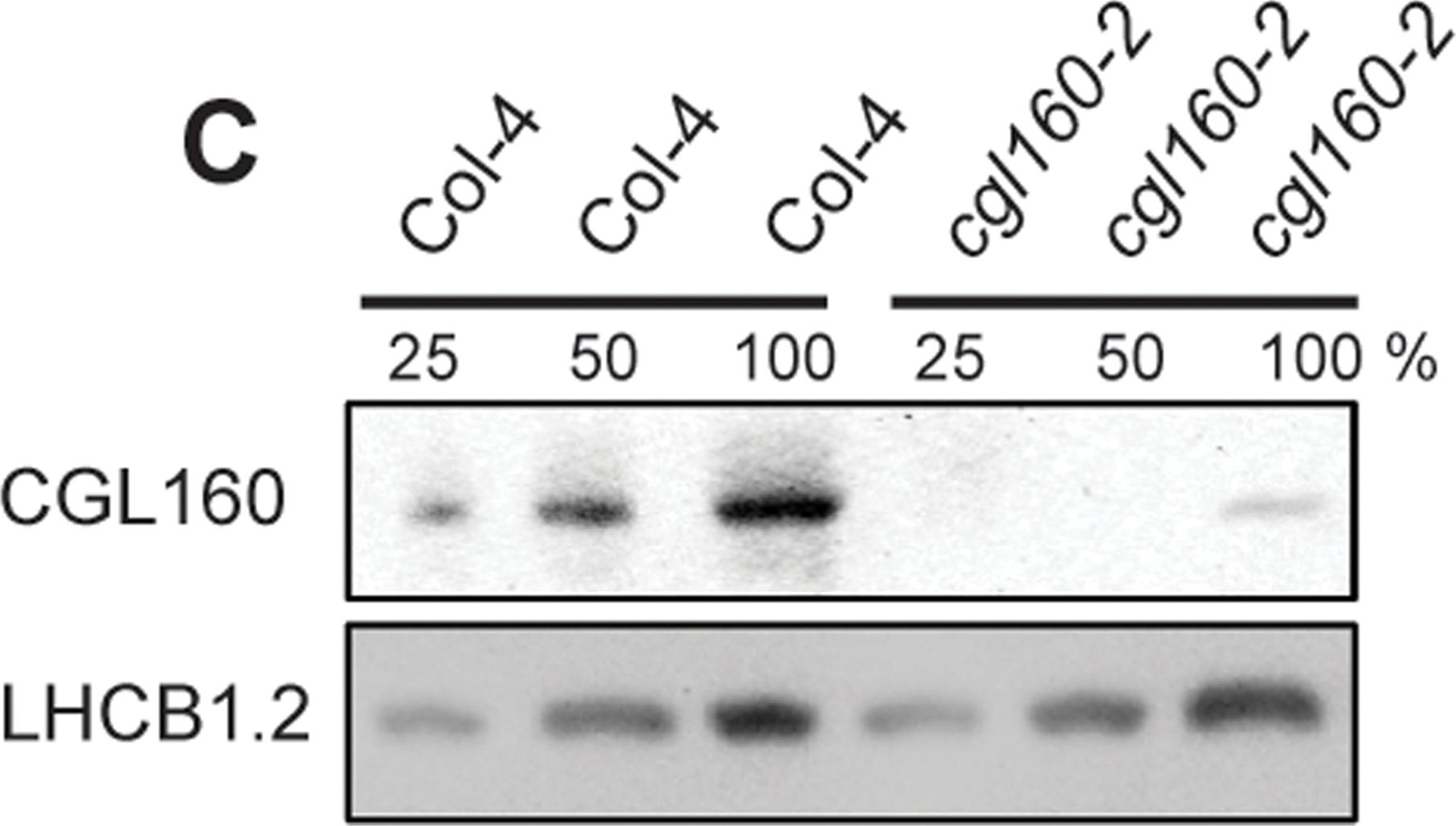
Open PublicationIsolation and characterization of Arabidopsis cgl160 mutants.A. DNA insertion sites in CGL160 gene of Arabidopsis. DNA insertion sites (black triangles) are shown in relation to the CGL160 gene structure. The two cgl160 alleles analyzed in this study are denoted as cgl160-1 and cgl160-2. The CGL160 coding region is indicated by the translational start (ATG). The CGL160 genomic locus contains nine exons but only the first four are shown in the fig (grey boxes), shown are also the first four introns (black thin connecting lines). Before ATG is the promoter region in light gray. The region used for CGL160 specific antibody is shown as antigen. B. Characterization of CGL160 amount in Arabidopsis cgl160-1 mutant from isolated chloroplasts. The CGL160 antibody was used for immunoblotting and 10 ?g protein was loaded in each lane. The LHCB1 antibody was used as a loading control. C. Characterization of CGL160 amount in Arabidopsis cgl160-2 mutant from isolated chloroplasts. The CGL160 antibody was used for immunoblotting and 10 ?g protein was loaded in each lane. The LHCB1 antibody was used as a loading control.
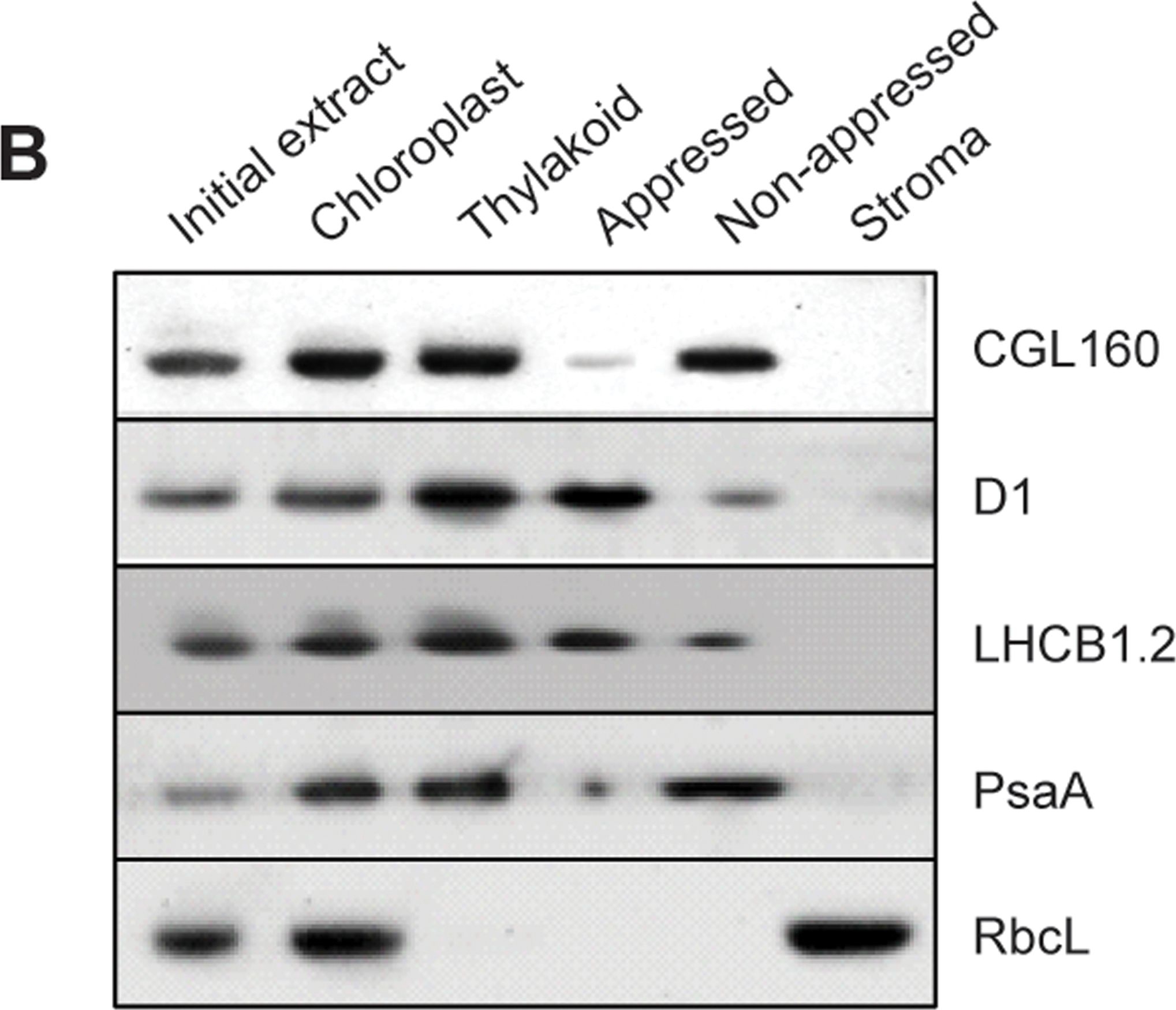
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 25835989
Journal: PLoS One
Figure Number: 3B
Published Date: 2015-04-04
First Author: Fristedt, R., Martins, N. F., et al.
Impact Factor: 2.942
Open PublicationCGL160 is a membrane-integral chloroplast protein located in non-appressed thylakoid membranes.A. Subcellular localization of CGL160. Immunoblot analysis performed on initial plant extract, isolated chloroplasts or isolated mitochondria. TOM40 is a marker for the mitochondrion. B. Suborganellar localization determined by immunoblot analysis of chloroplast proteins diagnostic for photosystem I (PsaA), photosystem II (D1), Rubisco, LHCII (LHCB1.2) and CGL160. Protein extracts were separated by SDS-PAGE and probed with specific antibodies directed against PsaA, (PSI reaction center subunit), D1 (PSII reaction center subunits), LHCB1 (outer PSII antenna protein), the large subunit of Rubisco, a soluble protein in the stroma, and CGL160. C. Immunoblot analysis show that CG160 is an integral membrane protein associated with the thylakoid membranes. Isolated thylakoid membranes were washed with 0.4 M NaCl, and the thylakoid membranes and the supernatant were probed by immunoblotting with antibodies against CGL160 and the PSII reaction center protein D1.
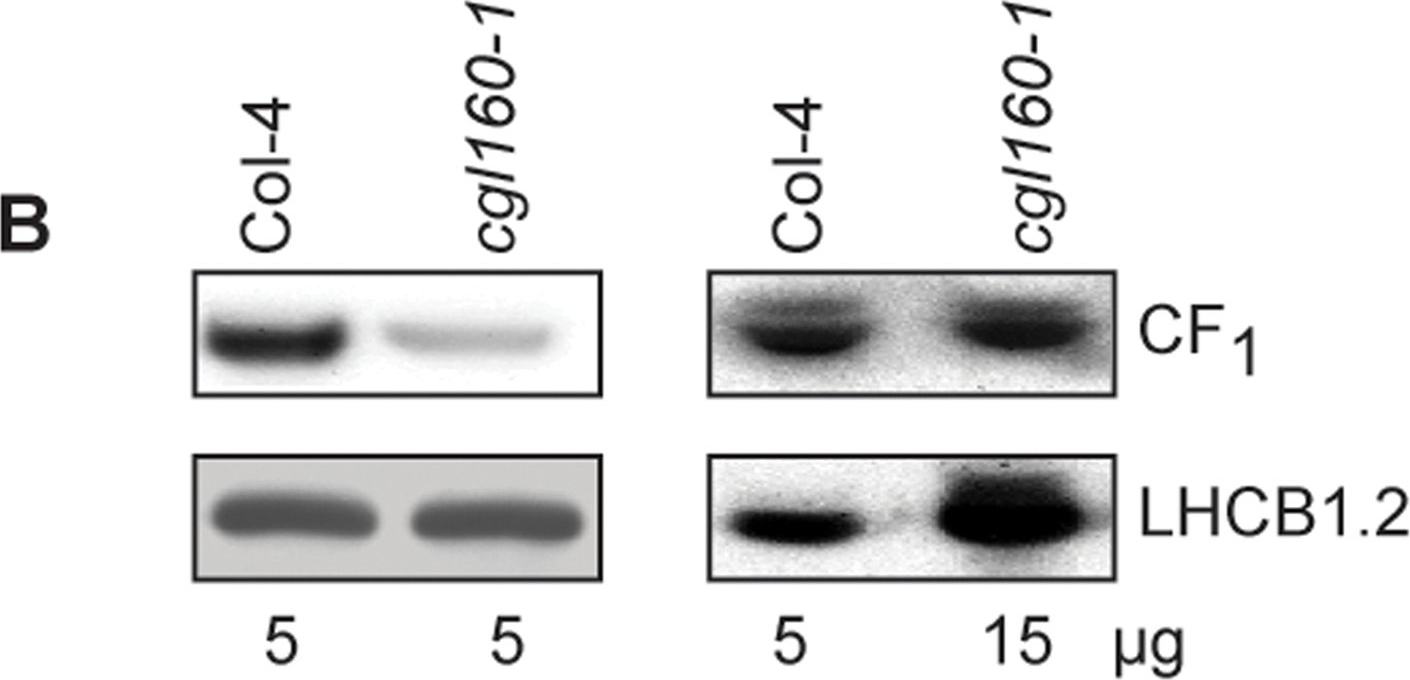
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 25835989
Journal: PLoS One
Figure Number: 7B
Published Date: 2015-04-04
First Author: Fristedt, R., Martins, N. F., et al.
Impact Factor: 2.942
Open PublicationAltered protein accumulation and stability of the chloroplast ATP synthase in the cgl160 mutant visualized by immunoblotting.A. Immunoblots with antibodies against essential subunits of the photosynthetic protein complexes of wild-type (Col-4) Arabidopsis and the two cgl160 T-DNA insertion lines grown under long-day and short-day conditions. Isolated thylakoid membranes were used, and equal amounts of chlorophyll were loaded onto the SDS-PAGE gel. For approximate quantification, wild-type samples from long-day plants were diluted to 10%, 25% and 50%, respectively. Accumulation of PSII was probed with antibodies against PsbB and PSBO. Additionally, the PSBS protein involved in NPQ and the minor PSII antenna protein LHCB4 were probed. Accumulation of the cytochrome b6f complex was probed with antibodies against the essential subunits PetA (cytochrome f), PetB (cytochrome b6), and PETC (Rieske protein). Accumulation of PSI was probed with antibodies against the reaction center subunit PsaB and the stromal ridge subunit PsaD. ATP synthase accumulation was probed with antibodies against the CF1 subunits AtpA (CF1?), AtpB (CF1?) and AtpD (CF1?) and antibodies against the CF0 subunits AtpF (CF0b) and AtpI (CF0a). B. Loading difference estimation for immunoblotting CF1 between wild type and cgl160-1. To obtain similar immunoblotting signal three times more (15 ?g protein) was needed for cgl160-1 compared to wild type (5 ?g protein). C. Maintenance of CF1 was measured by incubating leaves from wild type and cgl160-1 in solution containing the plastid protein synthesis inhibitor chloramphenicol for the indicated time points. Protein extract was isolated and separated by SDS-PAGE, immunoblotted and probed with specific antibodies against CF1 and LHCB2.1. Three times more protein was loaded from the mutant to obtain equal level of CF1 immunoblotting signal, as specified in B.
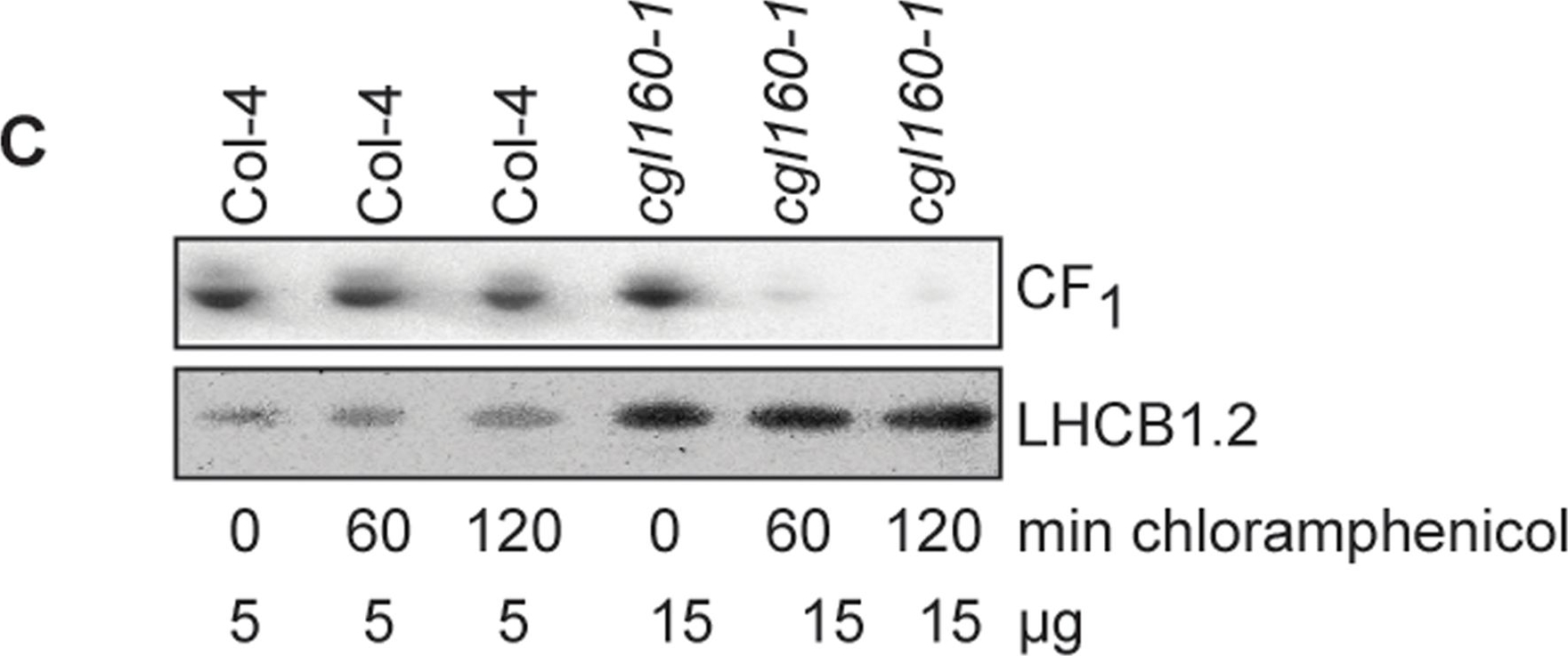
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 25835989
Journal: PLoS One
Figure Number: 7C
Published Date: 2015-04-04
First Author: Fristedt, R., Martins, N. F., et al.
Impact Factor: 2.942
Open PublicationAltered protein accumulation and stability of the chloroplast ATP synthase in the cgl160 mutant visualized by immunoblotting.A. Immunoblots with antibodies against essential subunits of the photosynthetic protein complexes of wild-type (Col-4) Arabidopsis and the two cgl160 T-DNA insertion lines grown under long-day and short-day conditions. Isolated thylakoid membranes were used, and equal amounts of chlorophyll were loaded onto the SDS-PAGE gel. For approximate quantification, wild-type samples from long-day plants were diluted to 10%, 25% and 50%, respectively. Accumulation of PSII was probed with antibodies against PsbB and PSBO. Additionally, the PSBS protein involved in NPQ and the minor PSII antenna protein LHCB4 were probed. Accumulation of the cytochrome b6f complex was probed with antibodies against the essential subunits PetA (cytochrome f), PetB (cytochrome b6), and PETC (Rieske protein). Accumulation of PSI was probed with antibodies against the reaction center subunit PsaB and the stromal ridge subunit PsaD. ATP synthase accumulation was probed with antibodies against the CF1 subunits AtpA (CF1?), AtpB (CF1?) and AtpD (CF1?) and antibodies against the CF0 subunits AtpF (CF0b) and AtpI (CF0a). B. Loading difference estimation for immunoblotting CF1 between wild type and cgl160-1. To obtain similar immunoblotting signal three times more (15 ?g protein) was needed for cgl160-1 compared to wild type (5 ?g protein). C. Maintenance of CF1 was measured by incubating leaves from wild type and cgl160-1 in solution containing the plastid protein synthesis inhibitor chloramphenicol for the indicated time points. Protein extract was isolated and separated by SDS-PAGE, immunoblotted and probed with specific antibodies against CF1 and LHCB2.1. Three times more protein was loaded from the mutant to obtain equal level of CF1 immunoblotting signal, as specified in B.
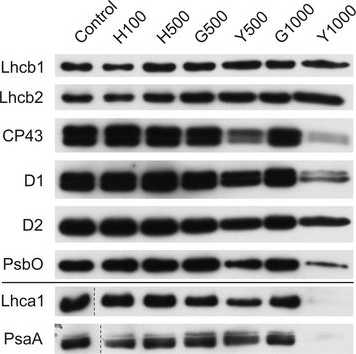
Reactant: Plant
Application: Western Blotting
Pudmed ID: 27590049
Journal: BMC Plant Biol
Figure Number: 9A
Published Date: 2016-09-02
First Author: Mazur, R., Sadowska, M., et al.
Impact Factor: 4.142
Open PublicationChanges of PSII and PSI antenna and core protein levels. Proteins from control and Tl-treated white mustard leaves were separated by SDS-PAGE followed by immunodetection with antibodies against Lhcb1, Lhcb2, Lhca1 (antenna proteins) and D1, D2, CP43, PsbO, PsaA (core proteins). Samples were loaded on the equal amount of chlorophyll (0.25 ?g). Description of samples abbreviation as given in the legend to Fig. 3
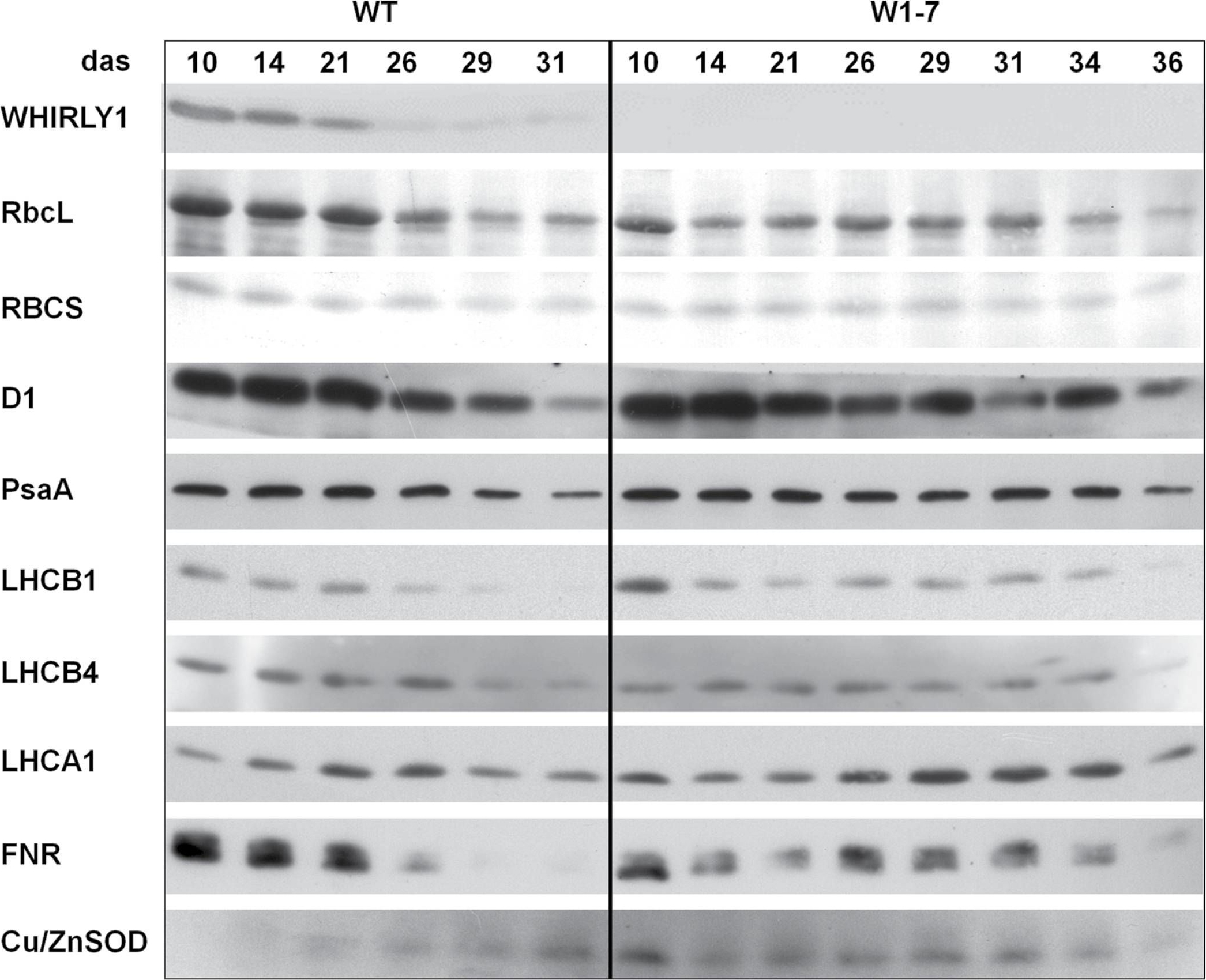
Reactant: Hordeum vulgare (Barley)
Application: Western Blotting
Pudmed ID: 28338757
Journal: J Exp Bot
Figure Number: 6A
Published Date: 2017-02-01
First Author: Kucharewicz, W., Distelfeld, A., et al.
Impact Factor: 6.088
Open PublicationImmunological analyses of the relative amounts of photosynthesis-associated proteins during senescence of primary leaves from wild-type (WT) and RNAi-W1-7 plants grown at high irradiance. Samples were prepared from wild-type plants grown for 10, 14, 21, 26, 29, and 31 das, as well as from RNAi-W1-7 plants grown for 10, 14, 21, 24, 29, 31, 34, and 36 das. The analysis was done with two biological replicates. WHIRLY1, photosynthesis-related proteins, and Cu/ZnSOD were detected by specific antibodies.
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 32963291
Journal: Sci Rep
Figure Number: 2C
Published Date: 2020-09-22
First Author: Wang, L., Leister, D., et al.
Impact Factor: 4.13
Open PublicationPerturbation of the RdDM pathway does not significantly affect chlorophyll biosynthesis. (a) Determination of total chlorophyll (Chl a?+?b) contents of 4-day-old seedlings grown under LD conditions. Chlorophyll was acetone-extracted and measured spectrophotometrically, and concentrations were determined as described (see “Methods”). Data are shown as mean values?±?SD from 6 different plant pools. Each pool contained more than 100 seedlings. Significant differences (t-test; p?
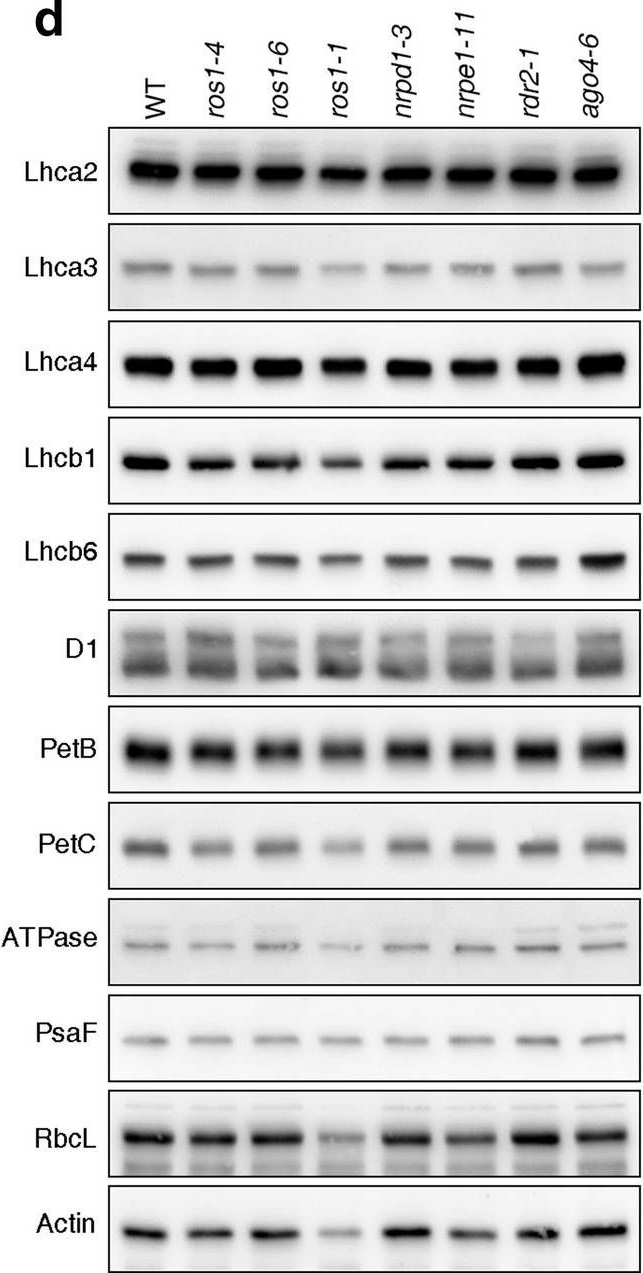
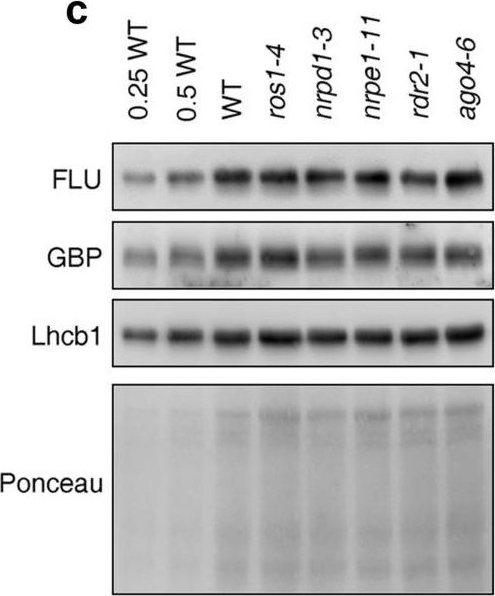
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 32963291
Journal: Sci Rep
Figure Number: 3D
Published Date: 2020-09-22
First Author: Wang, L., Leister, D., et al.
Impact Factor: 4.13
Open PublicationPhotomorphogenesis is not significantly affected in ros1, nrpd1, nrpe1, rdr2 or ago4 mutant seedlings. (a) Phenotypes (upper panel) and the corresponding maximum quantum yields of PSII (Fv/Fm) (lower panel) of 4-day-old etiolated seedlings. Fv/Fm was measured with an imaging Chl fluorometer (Imaging PAM). Scale bar?=?1 cm. (b) Phenotypes (upper panel) and corresponding Fv/Fm values (lower panel) of 3-day-old etiolated seedlings which had been exposed to continuous light for 24 h. (c) Immunoblot analysis of the PSII core proteins (D1 and D2), Lhcb1 and FLU during greening of etiolated seedlings. WT seedlings were grown for 3 days in the dark and exposed to light for between 0 and 48 h, as indicated. Extracted total proteins were normalized with respect to fresh weight and fractionated by SDS-PAGE. Blots were then probed with antibodies raised against the individual proteins. Total proteins from 5-day-old WT seedlings grown under continuous light (LL) and LD conditions (LD) were used as positive controls. The total protein accumulation of each sample was visualized by staining the gel with Coomassie Blue R250 (C.B.). The figure was assembled from different blots (delineated by a black rectangle) and full-length blots are presented in Supplementary Fig. S7. (d) Immunoblot analysis of representative photosynthesis proteins of 3-day-old etiolated mutant seedlings which had been exposed to continuous light for 24 h. Immunoblot analysis was performed as in (c). The figure was assembled from different blots (delineated by a black rectangle) and full-length blots are presented in Supplementary Fig. S8. (e) Real-time PCR analyses of 3-day-old etiolated WT (Col-0) and mutant seedlings that had been exposed to continuous light for 24 h. Real-time PCR was performed with primers specific for the nuclear genes LHCB1.2, LHCB2.1, LHCB6, LHCA5, PSBP-1 and PSBTn, and the plastid genes psaA and atpB. Note that the primers for LHCB2.1 also amplify LHCB2.2 mRNA. Expression values are reported relative to the corresponding transcript levels in the WT and were normalized with respect to the expression level of ACTIN2. Data are shown as mean values?±?SD from three different plant pools.
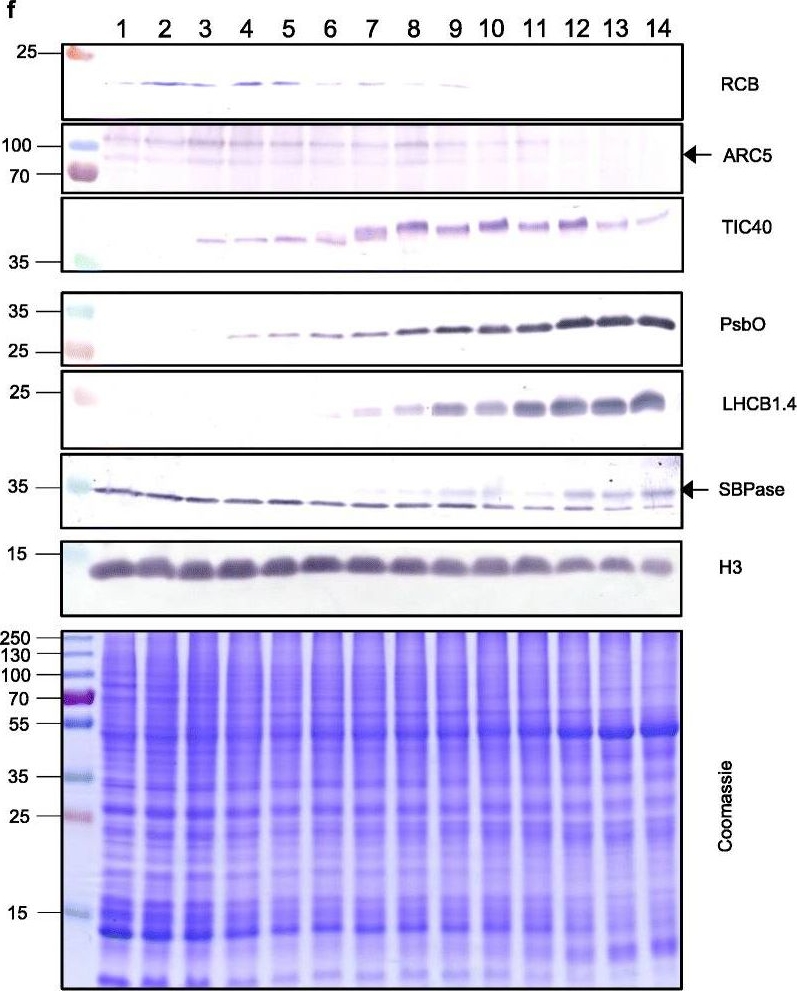
Reactant: Triticum aestivum (Common wheat)
Application: Western Blotting
Pudmed ID: 33975629
Journal: Genome Biol
Figure Number: 5F
Published Date: 2021-05-11
First Author: Loudya, N., Mishra, P., et al.
Impact Factor: 13.214
Open PublicationThe second, chloroplast growth phase involves greening and is supported by protein accumulation profiles. a Chlorophyll content, quantified in each of three independent biological replicates per sample. Error bars represent standard error of the mean. See Additional file 5: Table S6 for calculations. b, c, d Expression (Z-scores) of pigment biosynthesis and thylakoid biogenesis (b), light reactions (c) and carbon fixation-associated genes (d). e Expression (Z-scores) of chloroplast development-associated transcripts reflecting two stages of plastid development, peaking in the early plastid phase (RCB, ARC5 and TIC40) and, second, chloroplast phase (PSBO2, LHCB1.4 and SBPAse). f Immunoblot analysis of the protein products of the genes displayed in e. In total, 20??g of protein of samples 1–14 (for PSBO2, LHCB1.4 and SBPAse), 40??g (for RCB, ARC5 and TIC40) or 10??g (for Histone H3 as a constitutive control) was separated on denaturing SDS-PAGE gels, transferred to blots and probed with antibodies against the protein indicated. A Coomassie-stained total protein replica gel is also shown. Molecular weights (KDa) are indicated on the left. The results show one typical example from among three independent protein extraction and immunoblot experiments
- Additional Information
-
Additional information (application): Lhcb1 Protein is processed into mature form (Jansson 1999).
This product can be sold containing ProClin if requested - Background
-
Background: The major light-harvesting antenna complex II (LHCII) in photosynthetic eukaryotes is located in the thylakoid membrane of the chloroplast. It is a heterotrimeric complex formed by up to 3 different individual subtypes of chlorophyll a/b-binding proteins: Lhcb1, Lhcb2, and Lhcb3. Lhcb1 is the most abundant chlorophyll a/b-binding protein in eukaryotic phototrophs and often is coded by several nuclear genes.
A molecular characterisation of the LHCII proteins can be found in Caffarri et al. (2004) A Look within LHCII: Differential Analysis of the Lhcb1−3 Complexes Building the Major Trimeric Antenna Complex of Higher-Plant Photosynthesis. Biochemistry 43 (29): 9467–9476 - Product Citations
-
Selected references: Sulli et al. (2023). Generation and physiological characterization of genome‑edited Nicotiana benthamiana plants containing zeaxanthin as the only leaf xanthophyll. Planta . 2023 Oct 5;258(5):93. doi: 10.1007/s00425-023-04248-3.
Ye et al. (2023). The light-harvesting chlorophyll a/b-binding proteins of photosystem II family members are responsible for temperature sensitivity and leaf color phenotype in albino tea plant. J Adv Res . 2023 Dec 25:S2090-1232(23)00404-6.doi: 10.1016/j.jare.2023.12.017.
Hu et al. (2023). Drought affects both photosystems in Arabidopsis thaliana. New Phytol. 2023 Oct;240(2):663-675. doi: 10.1111/nph.19171. Epub 2023 Aug 2.
Hu C, Mascoli V, Elias E, Croce R. The photosynthetic apparatus of the CAM plant Tillandsia flabellate and its response to water deficit. J Plant Physiol. 2023 Mar;282:153945. doi: 10.1016/j.jplph.2023.153945
Jin et al. (2023) Dual roles for CND1 in maintenance of nuclear and chloroplast genome stability in plants. Cell Rep. 2023 Mar 28;42(3):112268. doi: 10.1016/j.celrep.2023.112268. Epub 2023 Mar 17.
Boussardon C, Carrie C, Keech O. Comparing plastid proteomes points towards a higher plastidial redox turnover in vascular tissues than in mesophyll cells. J Exp Bot. 2023 Apr 7:erad133. doi: 10.1093/jxb/erad133.
Harchouni et al. (2022) Guanosine tetraphosphate (ppGpp) accumulation inhibits chloroplast gene expression and promotes super grana formation in the moss Physcomitrium (Physcomitrella) patens. New Phytol. 2022;236(1):86-98. doi:10.1111/nph.18320
Gao Y et al. (2022) Chloroplast translational regulation uncovers nonessential photosynthesis genes as key players in plant cold acclimation. Plant Cell. 2022 Apr 26;34(5):2056-2079. doi: 10.1093/plcell/koac056. PMID: 35171295; PMCID: PMC9048916.
Ivanov et al. (2022) The decreased PG content of pgp1 inhibits PSI photochemistry and limits reaction center and light-harvesting polypeptide accumulation in response to cold acclimation. Planta 255, 36 (2022). https://doi.org/10.1007/s00425-022-03819-0
Medina-Puche et al (2021). Protocol for evaluating protein relocalization from the plasma membrane to chloroplasts. STAR Protoc. 2021 Sep 14;2(4):100816. doi: 10.1016/j.xpro.2021.100816. PMID: 34585156; PMCID: PMC8450296.
Chen, Liu & Liu (2021) Loss-Function of EGY1 Results in Photosynthesis Damage through Reducing Stability of Photosystem II in Arabidopsis thaliana. Russ J Plant Physiol (2021).
Wu et al. (2021). Formation of light-harvesting complex (LHC) II aggregates from LHCII-PSI-LHCI complexes in rice plants under high light. J Exp Bot. 2021 May 3:erab188. doi: 10.1093/jxb/erab188. Epub ahead of print. PMID: 33939808.
Loudya et al. (2021) Cellular and transcriptomic analyses reveal two-staged chloroplast biogenesis underpinning photosynthesis build-up in the wheat leaf. Genome Biol. 2021 May 11;22(1):151. doi: 10.1186/s13059-021-02366-3. PMID: 33975629; PMCID: PMC8111775.
Shukla et al. (2020). A novel method produces native LHCII aggregates from the photosynthetic membrane revealing their role in non-photochemical quenching. J Biol Chem. 2020 Oct 20:jbc.RA120.016181. doi: 10.1074/jbc.RA120.016181. Epub ahead of print. PMID: 33082138.
Zhu et al. (2020). A NAC transcription factor and its interaction protein hinder abscisic acid biosynthesis by synergistically repressing NCED5 in Citrus reticulata. J Exp Bot. 2020 Jun 22;71(12):3613-3625.doi: 10.1093/jxb/eraa118.
Forlani et al. (2020. HEBE, a novel positive regulator of senescence in Solanum lycopersicum. Sci Rep. 2020 Jul 3;10(1):11021.doi: 10.1038/s41598-020-67937-z.
Wang et al. (2020). Effects and Mechanisms of Foliar Application of Silicon and Selenium Composite Sols on Diminishing Cadmium and Lead Translocation and Affiliated Physiological and Biochemical Responses in Hybrid Rice (Oryza Sativa L.) Exposed to Cadmium and Lead. Chemosphere. 2020 Jul;251:126347. doi: 10.1016/j.chemosphere.2020.126347.
Galvis et al. (2020). H+ transport by K+ EXCHANGE ANTIPORTER3 promotes photosynthesis and growth in chloroplast ATP synthase mutants. Plant Physiol. pp.01561.2019. doi: 10.1104/pp.19.01561.
Averina et al. (2019). Photosynthesis and Oxygen Uptake Rate in Winter Rape Plants Treated with 5-Aminolevulinic Acid. Russian Journal of Plant Physiology volume 66, pages966–975(2019).
Koh et al. (2019). Heterologous synthesis of chlorophyll b in Nannochloropsis salina enhances growth and lipid production by increasing photosynthetic efficiency. Biotechnol Biofuels. 2019 May 14;12:122. doi: 10.1186/s13068-019-1462-3. eCollection 2019.
Rogowski et al. (2019). Photosynthesis and organization of maize mesophyll and bundle sheath thylakoids of plants grown in various light intensities. Environmental and Experimental Botany Volume 162, June 2019, Pages 72-86.
Lv et al. (2019). Uncoupled Expression of Nuclear and Plastid Photosynthesis-Associated Genes Contributes to Cell Death in a Lesion Mimic Mutant. Plant Cell. 2019 Jan;31(1):210-230. doi: 10.1105/tpc.18.00813.
Chen et al. (2018). TIC236 links the outer and inner membrane translocons of the chloroplast. Nature. 2018 Dec;564(7734):125-129. doi: 10.1038/s41586-018-0713-y.
Mao et al. (2018). Comparison on Photosynthesis and Antioxidant Defense Systems in Wheat with Different Ploidy Levels and Octoploid Triticale. Int J Mol Sci. 2018 Oct 2;19(10). pii: E3006. doi: 10.3390/ijms19103006.
Giovanardi et al. (2018). In pea stipules a functional photosynthetic electron flow occurs despite a reduced dynamicity of LHCII association with photosystems. Biochim Biophys Acta. 2018 May 24. pii: S0005-2728(18)30129-4. doi: 10.1016/j.bbabio.2018.05.013.
Myouga et al. (2018). Stable accumulation of photosystem II requires ONE-HELIX PROTEIN1 (OHP1) of the light harvesting-like family. Plant Physiol. 2018 Feb 1. pii: pp.01782.2017. doi: 10.1104/pp.17.01782.
Rantala et al. (2017). Proteomic characterization of hierarchical megacomplex formation in Arabidopsis thylakoid membrane. Plant J. 2017 Dec;92(5):951-962. doi: 10.1111/tpj.13732.
Shin et al. (2017), Complementation of a mutation in CpSRP43 causing partial truncation of light-harvesting chlorophyll antenna in Chlorella vulgaris. Sci Rep. 2017 Dec 20;7(1):17929. doi: 10.1038/s41598-017-18221-0.
Yang-Er Chen et al. (2017). Responses of photosystem II and antioxidative systems to high light and high temperature co-stress in wheat. J. of Exp. Botany, Volume 135, March 2017, Pages 45–55.
Mazur et al. (2016). Overlapping toxic effect of long term thallium exposure on white mustard (Sinapis alba L.) photosynthetic activity. BMC Plant Biol. 2016 Sep 2;16(1):191. doi: 10.1186/s12870-016-0883-4.
Kowalewska et al. (2016). Three-dimensional visualization of the internal plastid membrane network during runner bean chloroplast biogenesis. Dynamic model of the tubular-lamellar transformation. The Plant Cell March 21, 2016 tpc.01053.2015.
Fristedt et al. (2015). The thylakoid membrane protein CGL160 supports CF1CF0 ATP synthase accumulation in Arabidopsis thaliana. PLoS One. 2015 Apr 2;10(4):e0121658. doi: 10.1371/journal.pone.0121658.
Armbruster et al. (2014). Ion antiport accelerates photosynthetic acclimation in fluctuating light environments. Nat Commun. 2014 Nov 13;5:5439. doi: 10.1038/ncomms6439
- Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Oxygenic photosynthesis poster by prof. Govindjee and Dr. Shevela
Z-scheme of photosynthetic electron transport by prof. Govindjee and Dr. Björn and Dr. Shevela - Reviews:
-
Soo Yeon Ko | 2020-11-23We always use this antiboy whenever we want to the this band in Western blot. The result is very good! I can recommend this antibody :)Katya Georgieva | 2019-06-24The antibody is working on Haberlea rhodopensis thylakoid proteins (3 ug chlorophyll per lane) in concentration 1:1000. Development of Western blot: ECL. 2nd antibody dilution: 1:25000.Shizue Matsubara | 2012-04-04This antibody gave a clear single band with Arabidopsis leaf protein extracts.Maciej Garstka | 2009-03-19specific to pea, less to bean,spinach and rye
Accessories
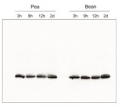
AS01 003 | Clonality: Polyclonal | Host: Rabbit | Reactivity: Photosynthetic eukaryotes including A. thaliana, A. hypogaea, B. sylvaticum, , C. arietinum, C. sinensis, C. quitensis Kunt Bartl, C. sativa, H. vulgare, C. reinhardtii, L. esculentum (Solanum lycopersicon), M. crystallinum, N. tabacum, O. sativa, P. patens, P. sativum, P. vulgaris, S. alba, S. oleracea, T. aestivum, Triticale, Z. mays