1
Anti-LSD1 | Lesion simulating disease 1 (rabbit antibody)
AS13 2746 | Clonality: Polyclonal | Host: Rabbit | Reactivity: Arabidopsis thaliana
- Product Info
-
Immunogen: KLH-conjugated synthetic peptide derived from Arabidopsis thaliana LSD1 sequence, UniProt:P94077, TAIR: AT4G20380
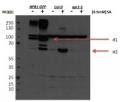
Host: Rabbit Clonality: Polyclonal Purity: Immunogen affinity purified serum in PBS pH 7.4. Format: Lyophilized Quantity: 50 µg Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Western blot (WB) Recommended dilution: 1 : 1000 (WB) Expected | apparent MW: 20 kD
- Reactivity
-
Confirmed reactivity: Arabidopsis thaliana Predicted reactivity: Brassica olereacea, Pisum sativum
Species of your interest not listed? Contact usNot reactive in: No confirmed exceptions from predicted reactivity are currently known - Application Examples
-
application example 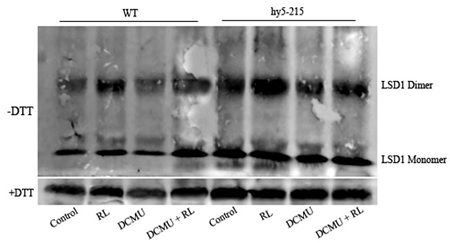
40 µg of total protein from 0.4g leaves extracted with1ml extraction buffer (50 mM Tris-HCl, pH 6.8, 50mM DTT, 4%SDS, 10%glycerol,PVPP) were separated on12 % SDS-PAGE using tank transfer and blotted 1h to PVDF. Blots were blocked with 5% skimmed milk powder for 1h at room temperature (RT) with agitation. Blot was incubated in the primary antibody at a dilution of 1: 1 000 for 4 h at RT with agitation. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 15 min and 3 times for 5 min in TBS-T at RT with agitation. Blot was incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, from ) diluted to 1:0 000 in for 1h at RT with agitation. The blot was washed as above and developed for 5 min with ECL according to the manufacturer's instructions. Exposure time was second.
Courtesy of Dr. Tingting Chai, South China Normal University, ChinaApplication examples: 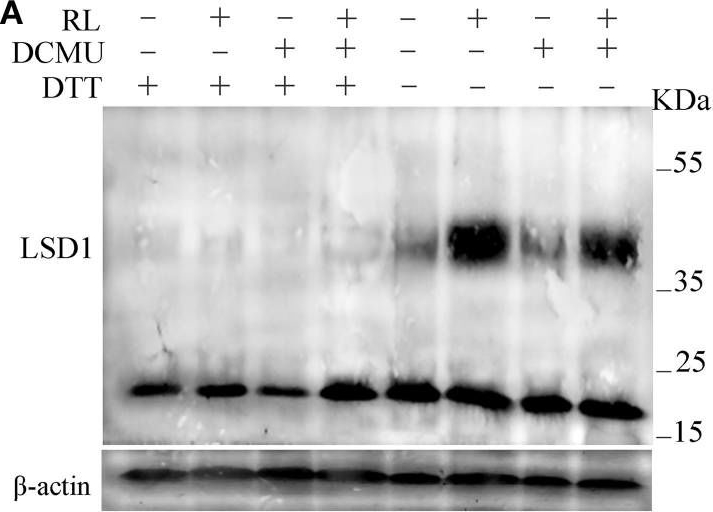
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 25999965
Journal: Front Plant Sci
Figure Number: 3A
Published Date: 2015-05-23
First Author: Chai, T., Zhou, J., et al.
Impact Factor: 5.435
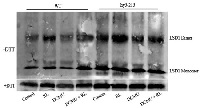
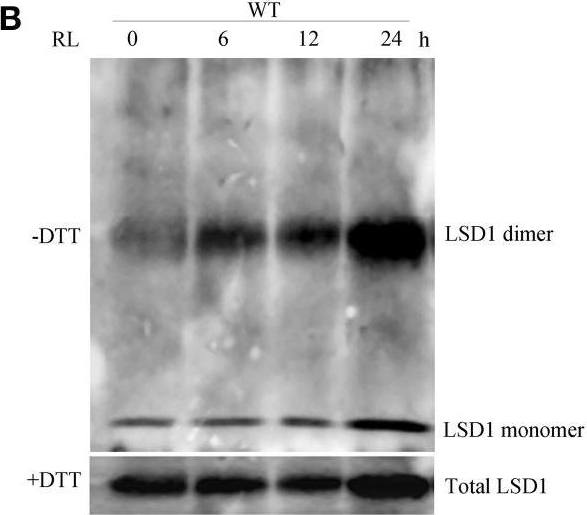
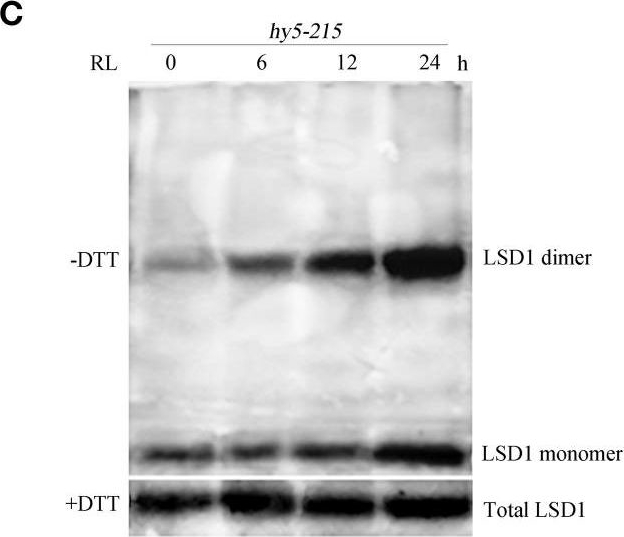
Open PublicationConformation of LSD1 was changed by immunoprecipitation assay and LSD1 activity was indirectly measured under RL. (A) Total protein extracted with (+) or without (–) DTT (50 mM) in the extraction buffer from control, RL, DCMU, and DCMU + RL treated WT was subjected to immunoprecipitation analysis, both dimmer, and monomer LSD1 protein were detected. (B) Total protein was extracted from WT leaves at times after RL treatment and analyzed by an immunoprecipitation assay. (C) The LSD1 conformation was detected in hy5-215 mutant. (D) The ratio of LSD1 dimer/monomer was analyzed quantitatively. (E) The CAT activity was measured in WT, lsd1-2 mutant, and hy5-215 mutant, the four-week old leaves were exposed to control, RL, DCMU and DCMU+ RL for 6 h. (F) The CAT activity of WT, lsd1-2 mutant, and hy5-215 mutant was detected at times after RL treatment. Different letters indicate statistically significant differences between treatments (Duncan's multiple range test: P < 0.05). Values represent means ± SD of three independent replicates.
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 25999965
Journal: Front Plant Sci
Figure Number: 3B
Published Date: 2015-05-23
First Author: Chai, T., Zhou, J., et al.
Impact Factor: 5.435
Open PublicationConformation of LSD1 was changed by immunoprecipitation assay and LSD1 activity was indirectly measured under RL. (A) Total protein extracted with (+) or without (–) DTT (50 mM) in the extraction buffer from control, RL, DCMU, and DCMU + RL treated WT was subjected to immunoprecipitation analysis, both dimmer, and monomer LSD1 protein were detected. (B) Total protein was extracted from WT leaves at times after RL treatment and analyzed by an immunoprecipitation assay. (C) The LSD1 conformation was detected in hy5-215 mutant. (D) The ratio of LSD1 dimer/monomer was analyzed quantitatively. (E) The CAT activity was measured in WT, lsd1-2 mutant, and hy5-215 mutant, the four-week old leaves were exposed to control, RL, DCMU and DCMU+ RL for 6 h. (F) The CAT activity of WT, lsd1-2 mutant, and hy5-215 mutant was detected at times after RL treatment. Different letters indicate statistically significant differences between treatments (Duncan's multiple range test: P < 0.05). Values represent means ± SD of three independent replicates.
Reactant: Arabidopsis thaliana (Thale cress)
Application: Western Blotting
Pudmed ID: 25999965
Journal: Front Plant Sci
Figure Number: 3C
Published Date: 2015-05-23
First Author: Chai, T., Zhou, J., et al.
Impact Factor: 5.435
Open PublicationConformation of LSD1 was changed by immunoprecipitation assay and LSD1 activity was indirectly measured under RL. (A) Total protein extracted with (+) or without (–) DTT (50 mM) in the extraction buffer from control, RL, DCMU, and DCMU + RL treated WT was subjected to immunoprecipitation analysis, both dimmer, and monomer LSD1 protein were detected. (B) Total protein was extracted from WT leaves at times after RL treatment and analyzed by an immunoprecipitation assay. (C) The LSD1 conformation was detected in hy5-215 mutant. (D) The ratio of LSD1 dimer/monomer was analyzed quantitatively. (E) The CAT activity was measured in WT, lsd1-2 mutant, and hy5-215 mutant, the four-week old leaves were exposed to control, RL, DCMU and DCMU+ RL for 6 h. (F) The CAT activity of WT, lsd1-2 mutant, and hy5-215 mutant was detected at times after RL treatment. Different letters indicate statistically significant differences between treatments (Duncan's multiple range test: P < 0.05). Values represent means ± SD of three independent replicates.
- Background
-
Background: LSD1 (Lesion simulating disease 1) is a negative regulator of reactive oxygen-induced cell death, cold stress-induced cell death, pathogen-induced hypersensitive response (HR), basal disease resistance. The protein is required for leaf acclimation in response to excess excitation energy.
Alternative names: CHILLING SENSITIVE 4, CHS4. - Product Citations
-
Selected references: Chai et al. (2015). LSD1 and HY5 antagonistically regulate red light induced-programmed cell death in Arabidopsis. Front Plant Sci. 2015 May 5;6:292. doi: 10.3389/fpls.2015.00292. eCollection 2015. - Protocols
-
- Reviews:
-
This product doesn't have any reviews.