1

1 | PEB (4x) | protein extraction buffer
AS08 300 | Extraction buffer for quantitative isolation of total soluble/membrane protein from plant tissue and algal/bacterial cells, optimized for subsequent western blot detection in denatured conditions
- Product Info
-
Quantity: 5 x 2 ml (4x stock) allows up to 75 isolations of plant material (using 500 µl 1x PEB for 100 mg fresh weight) or 190 isolations of algal material (using 200 µl 1x PEB for cell amounts corresponding to 4-10 µg total chlorophyll) Storage: Stable at RT for at least 1 month; short-term storage (6 monthss) at 4°C and long term storage (1 year or more) at -20°C. Tested applications: Protein extraction - Reactivity
-
Confirmed reactivity: PEB has been tested on a wide range of species and tissues from Higher plants, Mosses, Llichens, Algae, Diatoms, Dinoflagellates, Cyanobacteria. Extracts may be quantified using detergent (LDS) compatible methods, and have been shown to give highly reproducible and quantitative results in subsequent SDS PAGE gel electrophoresis, Western blotting, and Immunoprecipitation (IP).
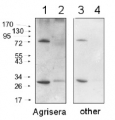
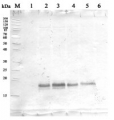
Most of Agrisera catalogue antibodies are tested on plant or algal samples extracted with this buffer. An example can be found here. - Application Examples
-
Before you start
Prepare sufficient 1x PEB for all samples by diluting 4x stock in sterile, deionized water (the pH of your 1x PEB should be between 8.25 and 8.75). It is recommended to include a protease inhibitor (not supplied with this buffer) from a freshly made stock while preparing the ready-to-use 1x PEB to increase the yield of non-degraded protein in the extract. We recommend including 1:50 vol/vol from a freshly prepared 50x stock (in 1x PEB) to give the desired final concentration recommended by the manufacturer (e.g. 0.1 mg/ml for Pefabloc SC, Roche).The total volume of 1x PEB required is dependent on the sample type and amount of tissue used: for 100 mg fresh plant tissue we recommend 500 µl 1x PEB; for algal samples (corresponding to 4-10 µg total chlorophyll) we recommend 200 µl 1x PEB. Keeping sample volumes in a range of 0.2-0.5 ml has been found to contribute to better extraction results, an upscale in volume is not recommended.
Material preparation
Plant tissue: weigh and snap freeze in liquid nitrogen and store at -80°C until processing.
Algal cultures: centrifuge to form a pellet or collect on filters (e.g.GF/F or polycarbonate) and freeze at -80°C until processing.
Extraction1
Grind frozen material in liquid N2 in a pre-chilled mortar with a pestle to a fine powder and transfer to a 1.5 ml tube
Keep material cool at any time during grinding, avoid spillage
2
Add 1x PEB and immediately freeze sample in liquid N2
500 µl for 100 mg plant tissue or 200 µl for cells corresponding to 4-10 µg total chlorophyll; keep tube upright to hold sample at the bottom of the tube
3
Carefully subject sample to sonication just until sample is thawn, re-freeze sample immediately in liquid N2 to avoid heating
Optimal results will be obtained using a microtip sonicator (e.g. Branson Ultrasonics Model 450)at low settings of about 30%; waterbath sonicators may also be used though this may lead to slightly less reproducible protein recovery rates;
4
Repeat sonication step (3) depending on species, place on ice until all samples are processed
For higher plants 2-3 cycles, for cyanobacteria 3 cycles, for Chlamydomonas 2 cycles, for Heterosigma, Thalassiosira and Trichodesmium 1 cycle
5
Centrifuge your samples for 3 min at 10 000 x g to remove insoluble material and unbroken cells, the pellet should be white/light-grey
An intense green color of the pellet indicates that disruption was not optimal and extraction conditions need to be adjusted (e,g, improved grinding and/or repeated sonication steps) using a new sample
6
Transfer supernatant to new tube using a pipette, be careful not carry over debris
Expect ~400 µl supernatant for the plant and ~150 µl for cyanobacterial/algal samples; collecting supernatant with a pipette as 2 x 200 (or 2 x 75 µl) reduces the risk of disturbing the pelleted debris
Protein determination
Assay total protein content of recovered supernatant using a detergent compatible assay. Based on the amount and/or tissue of the species used you may expect a protein content of 1.5-6 µg/µl.
Storage
Protein extracts may be stored for 24 hrs at +4°C or up to 6/12 month at -20°C/-80°C. We recommend to aliquot samples. Re-freezing protein samples may induce degradation/aggregation.
Loading on a gel
A freshly prepared reducing agent should be added (e.g. Dithiotreitol, final concentration 50 mM) to the volume prepared for loading. Heat at 70°C for 5 min, briefly spin down and load on a gel. Protein loads of 0.5-5 µg/lane should be sufficient for most Western Blot applications. - Additional Information
-
Additional information: Buffer components (4x): contains ~ 40% v/v glycerol [HOCH2CH(OH)CH2OH], Tris-HCl [NH2C(CH2OH)3 · HCl] pH 8.5, LDS [CH3(CH2)11OSO3Li], EDTA [(HO2CCH2)2NCH2CH2N(CH2CO2H)2]
It is recommended to include a protease inhibitor (not supplied with this buffer) from a freshly made stock while preparing the ready-to-use 1x PSB. Please, note that some proteins which are aimed to be analyzed can be subjected to proteosomal degradation. Therefore, inhibitors as MG132 must be added.
PEB has been optimized for quantitative small-scale preparation of whole protein extracts from plant/algal tissue. Extraction using the procedure described below will result in maximum yield of proteins and diminish protein degradation and aggregation.
Extracts may be quantified using detergent (LDS) compatible methods and have been shown to give highly reproducible and quantitative results in subsequent SDS PAGE gel electrophoresis, Western Blotting, and immunoprecipitation.
PEB has been tested on a wide range of species and tissues from higher plants, mosses, lichens, algae, diatoms, dinoflagellates, and cyanobacteria.
- Background
-
Background: PEB is an extraction buffer for disruption and solubilisation of total protein from plant tissue and algal cells. The use of the anionic detergent LDS together with one of the recommended procedure (combination of sonication and freeze/thaw cycles) has been shown to increase the number of solubilised and non-degraded proteins when compared to other methods of cell disruption (see reference). The estimated hands-on time for the recommended procedure is 20-30 minutes for 1-2 samples. Expected yields will be 1.5-6 µg/µl total protein (recovered from standard procedure) depending on the starting material, e.g. its biological stage, homogenization method used (bead beater vs. sonication).
- Product Citations
-
Selected references: Huang et al. (2023) Along with cyclic electron flow and non-photochemical quenching, water-water cycle is involved uniquely in alleviating Zn stress-caused photodamage in Melia azedarach. Tree Physiol. 2023 Apr 18:tpad045. doi: 10.1093/treephys/tpad045.
Altuntas et al. (2020). Proline-stimulated signaling primarily targets the chlorophyll degradation pathway and photosynthesis associated processes to cope with short-term water deficit in maize. Photosynth Res. 2020 Apr;144(1):35-48. doi: 10.1007/s11120-020-00727-w.
Pérez-López et al. (2020). Transcriptome Analysis Identifies Plasmodiophora brassicae Secondary Infection Effector Candidates. J Eukaryot Microbiol. 2020 Jan 11. doi: 10.1111/jeu.12784.
Morin et al. (2019). Morin et al. (2019). Response of the sea-ice diatom Fragilariopsis cylindrus to simulated polar night darkness and return to light. Limnology and Oceanography. 9999, 2019, 1â??20. (sea-ice diatom)
Bausch, A.R., Juhl, A.R., Donaher, N.A. et al. Mar Biol (2019) 166: 80.
Matsuo and Atsumi (2018). Xylosylation of proteins by expression of human xylosyltransferase 2 in plants. J Biosci Bioeng. 2018 Sep;126(3):371-378. doi: 10.1016/j.jbiosc.2018.03.013.
Brouwer et al. (2011) TheImpact ofLightIntensity onShade-InducedLeaf Senescence. Plant Cell Environ. Dec. 15 (ahead of print).
Kosawang et al. (2011) Hydrogen yield from a hydrogenase in Frankia R43 at different levels of the carbon source propionate. Journal of Environmental Management, Jan 26 - Protocols
-
Before you start | Prepare sufficient 1x PEB for all samples by diluting 4x stock in sterile, deionized water (the pH of your 1x PEB should be between 8.25 and 8.75). It is recommended to include a protease inhibitor (not supplied with this buffer) from a freshly made stock while preparing the ready-to-use 1x PEB to increase the yield of non-degraded protein in the extract. We recommend including 1:50 vol/vol from a freshly prepared 50x stock to give the desired final concentration recommended by the manufacturer (e.g. 0.1 mg/ml for Pefabloc SC, ROCHE). The total volume of 1x PEB required is dependent on the sample type and amount of tissue used: for 100 mg fresh plant tissue we recommend 450-500 µl 1x PEB; for algal samples (corresponding to 4-10 µg total chlorophyll) we recommend 200 µl 1x PEB. Keeping sample volumes in a range of 0.2-0.5 ml has been found to contribute to better extraction results, an upscale in volume is not recommended.
Preparation from plant tissue | weigh and snap freeze in liquid nitrogen and store at -80°C until processing. If possible, using fresh material is highly recommended.
Preparation from algal cultures | centrifuge to form a pellet or collect on filters (e.g.GF/F or polycarbonate) and freeze at -80°C until processing.
Extraction1Grind frozen material in liquid N2 in a pre-chilled mortar with a pestle to a fine powder and transfer to a 1.5 ml tubeKeep material cool at any time during grinding, avoid spillage and long waiting times2Add 1x PEB and immediately freeze sample in liquid N2500 µl for 100 mg plant tissue or 200 µl for cells corresponding to 4-10 µg total chlorophyll; keep tube upright to hold sample at the bottom of the tube3Carefully subject sample to extraction process by a method of choice. If it is sonication it requires to be performed just until sample is thawn, re-freeze sample immediately in liquid N2 to avoid heatingOptimal results will be obtained using a microtip sonicator (e.g. Branson Ultrasonics Model 450)at low settings of about 30%; waterbath sonicators may also be used though this may lead to slightly less reproducible protein recovery rates;4Repeat sonication step (3) depending on species, place on ice until all samples are processedFor higher plants 2-3 cycles, for cyanobacteria 3 cycles, for Chlamydomonas 2 cycles, for Heterosigma, Thalassiosira and Trichodesmium 1 cycle5Centrifuge your samples for 3 min at 10 000 x g to remove insoluble material and unbroken cells, the pellet should be white/light-greyAn intense green color of the pellet indicates that disruption was not optimal and extraction conditions need to be adjusted (e,g, improved grinding and/or repeated sonication steps) using a new sample6Transfer supernatant to new tube using a pipette, be careful not carry over debrisExpect ~400 µl supernatant for the plant and ~150 µl for cyanobacterial/algal samples; collecting supernatant with a pipette as 2 x 200 (or 2 x 75 µl) reduces the risk of disturbing the pelleted debris
Protein determination | assay total protein content of recovered supernatant using a detergent compatible assay for example BCA Protein Assay Kit (Pierce Prod. No. 23227), which is compatible with detergents and other components of extraction buffer. Based on the amount and/or tissue of the species used you may expect a protein content of 1.5-6 µg/ µl.Storage | protein extracts may be stored for 24 hrs at +4°C or up to 6/12 month at -20°C/-80°C. We recommend to aliquot samples. Re-freezing protein samples may induce degradation/aggregation.
Loading on a gel | a freshly prepared reducing agent should be added (e.g. Dithiotreitol, final concentration 50 mM) to the volume prepared for loading. Heat at 70°C for 5 min, briefly spin down and load on a gel. Protein loads of 0.5-5 µg/lane should be sufficient for most Western Blot applications.
Example of protein extraction protocol mortar and pestle | Total protein extracts from leaves of a model plant, Arabidopsis thaliana, wild type and mutants (NASC ID: N6192, N16366, N6244 and N16268, obtained from European Seed Stock Centre, http://arabidopsis.info/) were used for confirmation of antibody specificity (Table X). Mutant and wild type Arabidopsis thaliana plants were grown in a greenhouse at Umeå Plant Science Center (UPSC). Leaves from the five different plants were harvested and directly frozen in liquid nitrogen. The frozen leaves were grinded to fine powder with a pre-chilled pestle in a mortar containing liquid nitrogen. 100 mg powders were dissolved in 500 µl 1 x Protein Extraction Buffer (PEB) (Agrisera, Product AS08 300) with 1:50 complete, ULTRA Mini, EDTA-free, EASY pac protease inhibitor (Roche, Ref 0589279100). The samples were run in a BeadBeater (Retsch MM 400) at 25.0 Hz for 60 seconds. The beads were removed and the samples were centrifuged for 3 minutes at 13000 rpm. Afterwards the supernatants was transferred to new tubes and centrifuged again at 13000 rpm for 3 minutes followed by protein estimation analysis and freezing at -20⁰C. Protein concentration of the five different samples was measured using BCA Protein Assay Kit (Pierce Prod. No. 23227) .
Example of protein extraction protocol bead beater | The procedures for samples filtered onto glass fibre filters, or pelleted, are similar. 1. Add the target volume of extraction buffer. 2. Resuspend (or saturate the filter). 3. Flash freeze in liquid nitrogen. 4. Use the bead beater for the protocol defined time period. 5. For some recalcitrant samples the freeze/beat cycles are repeated.
Diatoms are can be easier to break than leaves due to the brittle glass wall and the cells are usually large. Protein determination in diatoms can be more difficult due to the presence of interfering materials.
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Agrisera Educational Poster focused on Protein Extracation - Reviews:
-
| 2011-06-30The extraction buffer works very well, but it is really somewhat expensive.Bastiaan Brouwer | 2008-12-04A bit expensive for its contents, but it works very well.