1
Anti-BIK1 | Botrytis-induced kinase 1
AS16 4030 | Clonality: Polyclonal | Host: Rabbit | Reactivity: Arabidopsis thaliana
- Product Info
-
Immunogen: KLH-conjugated synthetic peptide derived from Arabidopsis thaliana BIK1 sequence, UniProt: O48814, TAIR: AT2G39660
Host: Rabbit Clonality: Polyclonal Purity: Immunogen affinity purified serum in PBS pH 7.4. Format: Lyophilized Quantity: 50 µg Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Western blot (WB) Recommended dilution: 1 : 3000 (WB)
Expected | apparent MW: 44 kDa - Reactivity
-
Confirmed reactivity: Arabidopsis thaliana
Predicted reactivity: Noccaea caerulescens
Species of your interest not listed? Contact usNot reactive in: Brachypodium distachyon, Nicotiana benthamiana, Solanum lycopersicum
- Application Examples
-
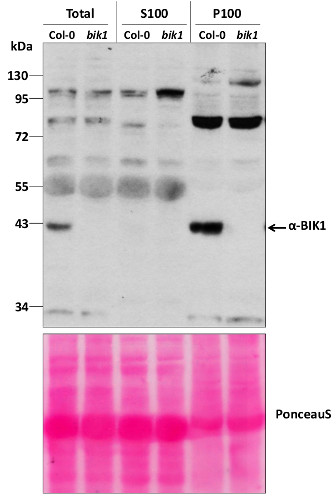
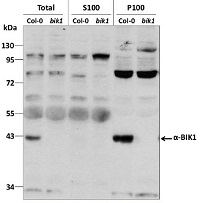
Total protein from 7-day old Arabidopsis thaliana seedlings Col-0 (wild-type) and bik1 (null mutant) (Lu et al, 2009) were extracted with 50mM HEPES-KOH buffer containing 250 mM sucrose, 5% glycerol, 50 mM NaPP, 1 mM NaMo, 25 mM NaF, 10mM EDTA, 0.5% PVP, 3mM DTT, 1mM PMSF, 10uM Leupeptin & 10nM Calyculin, and then fractionated by ultracentrifugation at 100,000 x gravity for 30 min at 4°C into soluble (S100) and microsomal (P100) proteins as described in LaMontagne et al. (2016). 30 µg proteins of Total, S100, and P100 fractions were denatured at 65°C for 5 min, separated on a 10 % SDS-PAGE and blotted 1h to nitrocellulose using tank transfer. Blots were blocked with 1x PBS (from Fisher Scientific BP665-1) + 0.1 %Tween 20 (PBS-T) + 5% milk for 1h at room temperature (RT) with agitation. Blot was incubated in the primary antibody at a dilution of 1: 1000 overnight at 4°C with agitation in 1x PBS-T + 5% milk. The antibody solution was decanted, and the blot was rinsed briefly once, then washed three times for 7 min in 1x PBS-T at RT with agitation. Blot was incubated with secondary antibody (Goat anti Rabbit IgG (H&L) –HRP conjugated from Agrisera (AS09 602) diluted to 1:10 000 in 1x PBS-T + 5% milk for 2 hr at RT with agitation. The blot was washed as above and developed for 5 min with Amersham ECL (RPN2106). Exposure time was 6 min.
Courtesy of Gayani Ekanayake & Dr. Antje Heese Division of Biochemistry, Interdisciplinary Plant Group (IPG) - University of Missouri; Columbia, MO, USA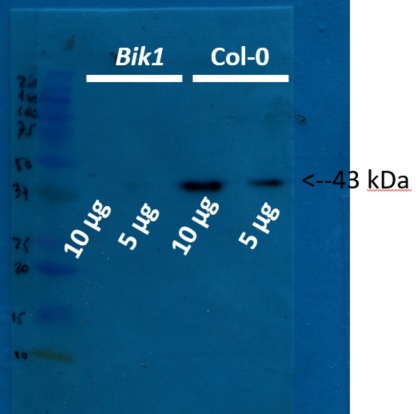
5 and 10 µg of protein from total plant extract of in vitro grown Arabidopsis thaliana Col0 and bik1 mutant were extracted using basic phenol protocol. 1g were grinded with liquid nitrogen with mortair and pestle, powder were resuspended in 3 mL of protein buffer (0.5 M Tris-HCl, 0.7 M sucrose, 1 mM PMSF, 50 mM EDTA, 0.1 M KCl and 0.2% β-mercaptoethanol; pH 8.0). Homogenate were centifuged at 8000 rpm and supernatant were mixed with three volumes of basic phenol, the mixture were incubated with agitation at room temperature during 10 mins. The mixture were centrifuged at 10000 rpm at 4ºC during 30 min, phenolic phase were recuperated in new tube and were mixed with one volume of ammonium acetate 0.1M in methanol, the mixture were incubated at -20 ºC during 4 hours. The mixture were centrifuged at 10000 rpm at 4º C during 20 minutes and protein pellet were washed three times with ammonium acetate 0.1M in methanol. Finally proteins were resuspended in tris-HCl (50 mM, pH 8.0) and the protein concentration were determined according Bradford method. Samples of 5 and 10µg were separated on 15 % SDS-PAGE using tank (BioRad system) to transfer to nitrocellulose membrane during 1 hour at 400 A. Blots were blocked with skimmed milk (10%) for 1h at room temperature (RT) with agitation. Blot was incubated in the primary antibody at a dilution of 1: 2 500 for 1h at RT with agitation. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 15 min and 3 times for 5 min in TBS-T at RT with agitation. Blot was incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, from Agrisera AS09 602) diluted to 1:20 000 in for 1h at RT with agitation. The blot was washed as above and developed for 5 min with ECL according to the manufacturer's instructions. Exposure time was 20 seconds. For transference control the membrane was stained with Ponceau red and integrity of proteins was evaluated using 12% SDS-PAGE comassie blue stained.
Courtesy of Dr. Rodrigo A. Contreras, Laboratorio de Fisiología y Biotecnología Vegetal Departamento de Biología, Facultad de Química y Biología, Universidad de Santiago de Chile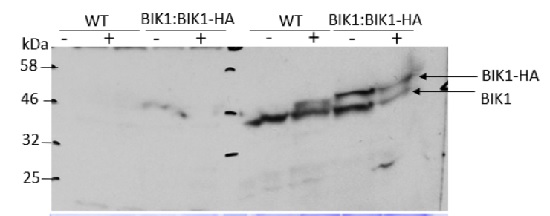
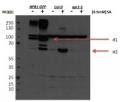
Two-week-old seedlings of Arabidopsis thaliana (grown under long day conditions) were infiltrated for 10 mins with flg22 at 1uM or mock, collected and frozen in liquid nitrogen. Approximately 1g of plant material was used for microsomal extraction according to the protocol described by LaMontage et al., 2016). Samples (60ul) were separated on 16 % SDS-PAGE and blotted 2h to PVDF using tank transfer. Blots were blocked with for 1h at room temperature (RT) with agitation. Blot was incubated in the primary antibody at a dilution of 1: 3 000 at 4C ON with agitation in TBS-T + 1% BSA. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 15 min and 3 times for 5 min in TBS-T at RT with agitation. Blot was incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, AS09 602, Agrisera) diluted to 1:20 000 in for 1h at RT with agitation. The blot was washed as above and developed for 2 mins with SuperSignal West Pico Chemiluminescent Substrate. Exposure time was ~ 1 hour.
Antibody is also recognizing phosphorylated form of BIK1. Applied treatment is decreasing pool of non-phosphorylated BIK1 and phosphoryled form is increasing.
Courtesy of Dr. Elwira Smakowska, Gregor Mendel Institute of Molecular Plant Biology , Vienna, Austria - Additional Information
-
Additional information: This product can be sold containing ProClin if requested Additional information (application): Antibody is also recognizng phosphorylated form of BIK1 - Background
-
Background: BIK1 (Botrytis-induced kinase 1) belongs to the protein kinase superfamily. BIK1 is a crucial component of host response signaling required to activate the resistance responses to Botrytis and A. brassicicola infection.
- Product Citations
-
Selected references: Toth et al. (2026). Motif-based substrate mapping of the receptor-like cytoplasmic kinase BIK1 reveals novel components and regulatory nodes of plant immunity. Nat Plants. 2026 Feb 9. doi: 10.1038/s41477-025-02218-z.
Salguero-Linares et al. (2025). Lack of AtMC1 catalytic activity triggers autoimmunity dependent on NLR stability. EMBO Rep . 2025 May;26(9):2378-2412. doi: 10.1038/s44319-025-00426-4.
Ngou et al. (2021) Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature. 2021 Mar 10. doi: 10.1038/s41586-021-03315-7. Epub ahead of print. PMID: 33692545.
Wang et al. (2021) Arabidopsis PUB2 and PUB4 connect signaling components of pattern-triggered immunity. New Phytol. 2021 Dec 17. doi: 10.1111/nph.17922. Epub ahead of print. PMID: 34918346 - Protocols
-
- Reviews:
-
This product doesn't have any reviews.
Accessories
AS10 687 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A. thaliana, H. vulgare, N. bentamiana, S. oleracea, S. lycopersicum, T. aestivum, Z. mays, V. vinifera