1
Anti-HydA | Iron-hydrogenase HydA1/HydA2
AS09 514 | Clonality: Polyclonal | Host: Rabbit | Reactivity: Chlamydomonas reinhardtii
- Product Info
-
Immunogen: Recombinant, full length Chlamydomonas reinhardtii HydA-2 Q8VZZ0
Host: Rabbit Clonality: Polyclonal Purity: Serum Format: Lyophilized Quantity: 50 µl Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Western blot (WB) Recommended dilution: 1 : 5000 (WB) Expected | apparent MW: 53.7 | 48 kDa (after transit peptide is cleaved) - Reactivity
-
Confirmed reactivity: Chlamydomonas reinhardtii Predicted reactivity: Ostreococcus sp.
Species of your interest not listed? Contact usNot reactive in: No confirmed exceptions from predicted reactivity are currently known - Application Examples
-
Application example
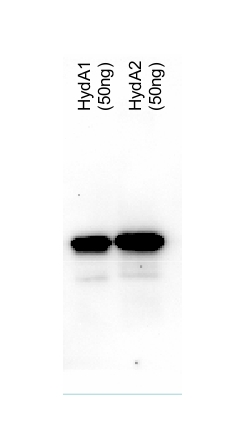
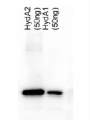
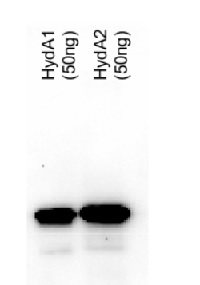
50ng of purified protein (HydA1 and HydA2) were separated on 10% SDS-PAGE and blotted 25 min to PVDF membrane. Filters were blocked 1h with 3% low-fat milk powder in PBS-T (0.1% TWEEN 20) and probed with anti-HydA1/2 (AS09 514, 1:5000, over night at 4°C) and secondary anti-rabbit (1:10 000, 1 h) antibody (HRP conjugated, manufacture Pierce) in PBS-T containing 3% low fat milk powder. Antibody incubations were followed by washings in PBS-T (10, +10min and PBS (+5, +5 min). All washing steps were performed at RT with agitation. Signal was detected with ECL (Millipore) using CCD camer. Exposure time was 20 min.
The heterolog expressed proteins have both calculated MWs of 51 kDa (due to the tag) and run according to their size.
Courtesy Dr. Thomas Happe, Ruhr-Univer, Germany
- Additional Information
-
Additional information: In Chlamydomonas HydA is present in low levels of 1 µg/liter of culture. Therefore, an induction of cells by anaerobic adaptation or sulfur depravation (10 x higher amount than with anaerobic adaptation) is necessary for successful detection using this antibody. Methods of HydA induction are described in Hemschemeier et al. 2009.
To detect HydA1/A2 in Chlamydomonas extracts amount loaded per well corresponds to 2 µg of chlorophyll for sulfur deprived cells, where relatively much HydA1 is synthesized or corresponds to 2-4 µg of artificially anaerobic induced cultures, where the HydA1 protein level is lower. This antibody is recognizing 1 ng of recombinant HydA protein.Additional information (application): HydA1 (497aa) has a calculated MW of 53.1 kDa, but this is including the signal peptide, which gets cleaved off. The protein without TP has a calculated MW of 47.5 kDa and runs according to its size at about 48 kDa.
HydA2 (505aa) has a calculated MW of 53.7 kDa, but this is including the signal peptide, which gets cleaved off. The protein without TP can only be estimated, since the cleavage site is known only from in silico analysis. It has a calculated MW of 47.3 kDa and should run in the gel also according to its size.
This antibody is binding recombinant HydA1/2 protein. - Background
-
Background: Iron-hydrogenase HydA2 is catalyzing reversible oxidation of molecular hydrogen. In Chlamydomonas the protein is present in low levels of 1 µg/liter of culture.Synonyme: HYD1/1
- Product Citations
-
Selected references: Nagy et al. (2023). Photoautotrophic and sustained H2 production by the pgr5 mutant of Chlamydomonas reinhardtii in simulated daily light conditions. International Journal of Hydrogen Energy Volume 53, 31 January 2024, Pages 760-769.
Grinberg et al (2023) Peptide self-assembly as a strategy for facile immobilization of redox enzymes on carbon electrode
Jokel et al. (2020). Elimination of the flavodiiron electron sink facilitates long-term H2 photoproduction in green algae. Biotechnol Biofuels. 2019 Dec 5;12:280. doi: 10.1186/s13068-019-1618-1.
Lindblad et al. (2019). CyanoFactory, a European consortium to develop technologies needed to advance cyanobacteria as chassis for production of chemicals and fuels. Algal Research, Volume 41, August 2019, 101510.
Weiner at al (2018), Overcoming the expression barrier of the ferredoxin‑hydrogenase chimera in Chlamydomonas reinhardtii supports a linear increment in photosynthetic hydrogen output. Algal Research Volume 33, July 2018, Pages 310-315
Wei et al. (2017). Light Intensity is Important for Hydrogen Production in NaHSO3-Treated Chlamydomonas reinhardtii. Plant Cell Physiol. 2017 Mar 1;58(3):451-457. doi: 10.1093/pcp/pcw216.
Eilenberg et al. (2016). The dual effect of a ferredoxin-hydrogenase fusion protein in vivo: successful divergence of the photosynthetic electron flux towards hydrogen production and elevated oxygen tolerance. Biotechnol Biofuels. 2016 Aug 30;9(1):182. doi: 10.1186/s13068-016-0601-3. eCollection 2016.
Liran et al. (2016). Microoxic Niches within the Thylakoid Stroma of Air-Grown Chlamydomonas reinhardtii Protect [FeFe]-Hydrogenase and Support Hydrogen Production under Fully Aerobic Environment. Plant Physiol. 2016 Sep;172(1):264-71. doi: 10.1104/pp.16.01063. Epub 2016 Jul 21.
Reifschneider-Wegner et al. (2014). Expression of the [FeFe] hydrogenase in the chloroplast of Chlamydomonas reinhardtii. Int J of Hydrogen Energy, Volume 39, Issue 8, 6 March 2014, Pages 3657–3665.
Pinto et al. (2013). Rubisco mutants of Chlamydomonas reinhardtii enhance photosynthetic hydrogen production. Appl Microbiol Biotechnol. May 7.
Magneschi et al. (2012). A Mutant in the ADH1 Gene of Chlamydomonas reinhardtii Elicits Metabolic 2 Restructuring during Anaerobiosis. Plant Physiol. January 23 (ahead of print). - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Agrisera Educational Posters Collection - Reviews:
-
This product doesn't have any reviews.