1
Anti-RA | Rubisco activase
AS10 700 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A. thaliana, Caesalpinia pulcherrima, C. sativa, C. reinhardtii, H. spontaneum, F. pRatensis, G. max, G. hirsutum, G. barbadense, L. perenne, N. oceanica, N. tabacum, O. sativa, P. balsamifera, R. discolor, S. lycopersicum, Z. mays, T. salsuginea, red sulfur bacterium T. sp. Cad16 (isolated from Lake Cadagno)
Replaced by AS22 4868
- Product Info
-
Immunogen: Purified, recombinant Rubisco activase from Gossypium hirsutum Q9AXG1
Host: Rabbit Clonality: Polyclonal Purity: Serum Format: Lyophilized Quantity: 50 µl Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Western blot (WB) Recommended dilution: 1 : 5000-1 : 10 000 (WB) Expected | apparent MW: 47 and 42 kDa (maize, tobacco, Chlamydomonas)
- Reactivity
-
Confirmed reactivity: Arabidopsis thaliana, Camelina sativa, Chlamydomonas reinhardtii, Caesalpinia pulcherrima, Hordeum spontaneum, Festuca pratensis, Glycine max, Gossypium hirsutum, Gossypium barbadense, Kalanchoë fedtschenkoi, Lolium perenne, Nannochloropsis oceanica, Nicotiana tabacum, Oryza sativa, Populus balsamifera, Rhoeo discolor, Solanum lycopersicum, Zea mays, Thellungiella salsuginea, red sulfur bacterium Thiodictyon sp. Cad16 (isolated from Lake Cadagno) Predicted reactivity: Glycine max, Gossypium mexicanum, Hordeum vulgare, Medicago sativa, Olea europea, Picea sitcHensis, Physcomitrium patens, Ricinus communis, Solanum lycopersicum, Spinacia oleracea, Triticum aestivum, Zea mays, Vitis vinifera
Species of your interest not listed? Contact usNot reactive in: marine picocyanobacteria - Application Examples
-
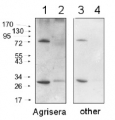
3, 5 and 11 μg of total soluble protein from Arabidopsis thaliana (1), Oryza sativa (2) and Camelina sativa (3) extracted with 50 mM Tricine-NaOH, pH 8, 10 mM EDTA, 1% PVP-40, 20 mM β-mercaptoethanol, 1 mM PMSF and 10 μM leupeptin were separated on 12 % SDS-PAGE and blotted 1h to PVDF. Blots were blocked with 4% non-fat milk in TBS for 1h at room temperature (RT) with agitation. Blot was incubated in the primary antibody at a dilution of 1: 10 000 for over night with agitation. The antibody solution was decanted and the blot was rinsed briefly with H2O, then washed six times for 15 min in TBS-T at RT with agitation. Blot was incubated in secondary antibody (anti-rabbit IgG alkaline phosphatase conjugated) diluted to 1:3000 in 0.5% non-fat milk in TBS for 2h at RT with agitation. The blot was washed with four changes of TBS-T and developed for 5 min with NBT/BCIP according to the manufacturer’s instructions (Promega). There are two forms of activase (alpha and beta). Alpha is about 46-47 Kda, beta is about 42 kDa what is shown on the blot above.
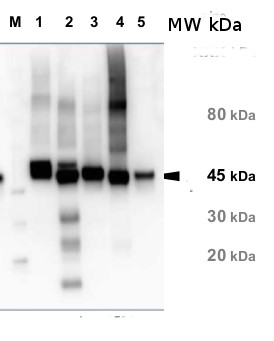
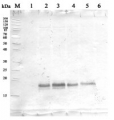
5 µg of total protein from samples such as Arabidopsis thaliana leaf (1) , Hordeum vulgare leaf (2), Zea mays leaf (3), Nicotiana tabacum (4), Chlamydomonas reinhardtii total cell (5), were extracted with Protein Extraction Buffer PEB (AS08 300). Samples were diluted with 1X sample buffer (NuPAGE LDS sample buffer (Invitrogen) supplemented with 50 mM DTT and heat at 70°C for 5 min and keept on ice before loading. Protein samples were separated on 4-12% Bolt Plus gels, LDS-PAGE and blotted for 70 minutes to PVDF using tank transfer. Blots were blocked immediately following transfer in 2% blocking reagent or 5% non-fat milk dissolved in 20 mM Tris, 137 mM sodium chloride pH 7.6 with 0.1% (v/v) Tween-20 (TBS-T) for 1h at room temperature with agitation. Blots were incubated in the primary antibody at a dilution of 1: 10 000 (in blocking reagent) for 1h at room temperature with agitation. The antibody solution was decanted and the blot was rinsed briefly twice, and then washed 1x15 min and 3x5 min with TBS-T at room temperature with agitation. Blots were incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, recommended secondary antibody AS10 1489, Agrisera) diluted to 1:25 000 in blocking reagent for 1h at room temperature with agitation. The blots were washed as above. The blot was developed for 5 min with chemiluminescent detection reagent according the manufacturers instructions. Images of the blots were obtained using a CCD imager (VersaDoc MP 4000) and Quantity One software (Bio-Rad). Exposure time was 30 seconds.Application examples: 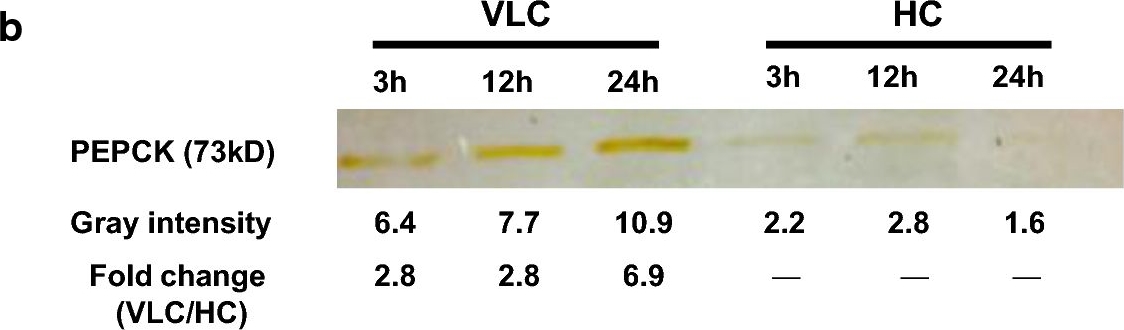
Reactant: Nannochloropsis gaditana
Application: Western Blotting
Pudmed ID: 31297156
Journal: Biotechnol Biofuels
Figure Number: 6B
Published Date: 2019-07-13
First Author: Wei, L., El Hajjami, M., et al.
Impact Factor: 5.839
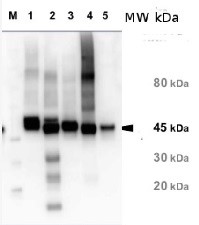
Open PublicationEnzymatic assays of key biochemical CCM genes in N. oceanica under VLC/HC. a Enzyme activities of C4-like genes under VLC and HC, including ME, MDH, PEPC and PEPCK. b Quantification of PEPCK protein under VLC and HC. Immunoblot band was detected from the cellular extract under VLC but not under HC (loading of 15 ?g total protein)
- Additional Information
-
Additional information: This product can be sold containing ProClin if requested Additional information (application): There are two forms of activase (alpha and beta) in some species (for example Arabidopsis, camelina, spinach, rice) and only one form in other species (tobacco, maize, Chlamydomonas). Alpha is about 46-47 Kda, beta is about 42 kDa. Species that have only one form have the beta form.
This product can be sold containing ProClin if requested - Background
-
Background: RA - Ribulose bisphosphate carboxylase/oxygenase activase is an enzyme localized to chloroplasts which activates Rubisco by promoting ATP-dependent conformational changes. Alternative name: RuBisCo activase RCA.
- Product Citations
-
Selected references: Chao et al. (2024). Molecular characterization and expression pattern of Rubisco activase gene GhRCAβ2 in upland cotton (Gossypium hirsutum L.). Genes Genomics. 2024 Apr;46(4):423-436. doi: 10.1007/s13258-024-01494-x.
Fukushi et al. (2024). Overexpression of thioredoxin-like protein ACHT2 leads to negative feedback control of photosynthesis in Arabidopsis thaliana J Plant Res. 2024 Feb 17.doi: 10.1007/s10265-024-01519-2.
Thagun et al. (2022) Non-transgenic Gene Modulation via Spray Delivery of Nucleic Acid/Peptide Complexes into Plant Nuclei and Chloroplasts. ACS Nano. 2022 Mar 22;16(3):3506-3521. doi: 10.1021/acsnano.1c07723. Epub 2022 Feb 23. PMID: 35195009; PMCID: PMC8945396.
Cao et al. (2022) Autophagic pathway contributes to low-nitrogen tolerance by optimizing nitrogen uptake and utilization in tomato. Hortic Res. 2022 Mar 23;9:uhac068. doi: 10.1093/hr/uhac068. PMID: 35669705; PMCID: PMC9164271.
Amiya et al. (2021) Membrane DnaJ-Like Chaperone with Oxidizing Activity in Chlamydomonas reinhardtii. Int J Mol Sci. 2021 Jan 24;22(3):1136. doi: 10.3390/ijms22031136. PMID: 33498879; PMCID: PMC7865324.
Oikawa et al. (2021) Mitochondrial movement during its association with chloroplasts in Arabidopsis thaliana. Commun Biol. 2021 Mar 5;4(1):292. doi: 10.1038/s42003-021-01833-8. PMID: 33674706.
Wang et al. (2021) Insights Into the Gene Regulation in Jasmonate-Induced Whole-Plant Senescence of Tobacco Under Non-Starvation Condition. Plant Cell Physiol. 2021 Sep 15:pcab140. doi: 10.1093/pcp/pcab140. Epub ahead of print. PMID: 34523687.
Trojak et al. (2021) Effects of partial replacement of red by green light in the growth spectrum on photomorphogenesis and photosynthesis in tomato plants. Photosynth Res. 2021 Sep 27. doi: 10.1007/s11120-021-00879-3. Epub ahead of print. PMID: 34580802.
Yokochi et al.(2021) Oxidative regulation of chloroplast enzymes by thioredoxin and thioredoxin-like proteins in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2021 Dec 21;118(51):e2114952118. doi: 10.1073/pnas.2114952118. PMID: 34907017; PMCID: PMC8713810.
Suganami et al. (2020). Effects of Overproduction of Rubisco Activase on Rubisco Content in Transgenic Rice Grown at Different N Levels. Int J Mol Sci. 2020 Feb 27;21(5). pii: E1626. doi: 10.3390/ijms21051626.
Salesse-Smith et al. (2018). Overexpression of Rubisco subunits with RAF1 increases Rubisco content in maize. Nat Plants. 2018 Oct;4(10):802-810. doi: 10.1038/s41477-018-0252-4.
Yoshida et al. (2018). Thioredoxin-like2/2-Cys peroxiredoxin redox cascade supports oxidative thiol modulation in chloroplasts. Proc Natl Acad Sci U S A. 2018 Aug 13. pii: 201808284. doi: 10.1073/pnas.1808284115.
Tamburino et al. (2017). Chloroplast proteome response to drought stress and recovery in tomato (Solanum lycopersicum L.). BMC Plant Biol. 2017 Feb 10;17(1):40. doi: 10.1186/s12870-017-0971-0.
Wei et al. (2017). Enhancing photosynthetic biomass productivity of industrial oleaginous microalgae by overexpression of RuBisCO activase. Algal Research Volume 27, November 2017, Pages 366-375.
Yin et al. (2016). Interplay between mitogen-activated protein kinase and nitric oxide in brassinosteroid-induced pesticide metabolism in Solanum lycopersicum. J Hazard Mater. 2016 Oct 5;316:221-31. doi: 10.1016/j.jhazmat.2016.04.070. Epub 2016 Apr 29.
Hu et al. (2015). Site-specific Nitrosoproteomic Identification of Endogenously S-Nitrosylated Proteins in Arabidopsis. Plant Physiol. 2015 Feb 19. pii: pp.00026.2015.
Jurczyk et al. (2015). Evidence for alternative splicing mechanisms in meadow fescue (Festuca pratensis) and perennial ryegrass (Lolium perenne) Rubisco activase gene. J Plant Physiol. 2014 Dec 18;176C:61-64. doi: 10.1016/j.jplph.2014.11.011.
Jedmowski et al. (2014). Comparative analysis of drought stress effects on photosynthesis of Eurasian and North African genotypes of wild barley. Photosynthetica, September 2014.
Yin et al. (2014). Characterization of Rubisco activase genes in maize: an alfa-isoform gene functions alongside a Beta-isoform gene. Plant Physiol. 2014 Apr;164(4):2096-106. doi: 10.1104/pp.113.230854. Epub 2014 Feb 7.
Wiciarz et al. (2014). Enhanced chloroplastic generation of H2 O2 in stress-resistant Thellungiella salsuginea in comparison to Arabidopsis thaliana. Physiol Plant. 2014 Jun 24. doi: 10.1111/ppl.12248.
Chen et al. (2013). Physiological Mechanisms for High Salt Tolerance in Wild Soybean (Glycine soja) from Yellow River Delta, China: Photosynthesis, Osmotic Regulation, Ion Flux and antioxidant Capacity. PLoS One. 2013 Dec 12;8(12):e83227. doi: 10.1371/journal.pone.0083227. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Agrisera Educational Posters Collection
- Reviews:
-
Saskia Zeilfelder | 2022-08-16We analyzed whole cell samples of Chlamydomonas reinhardtii using 10 µg total protein or 1 µg total chlorophyll per lane. After WB transfer we used an AB dilution of 1:10.000. The product worked really well and we obtained signals at approximately 45 kDa.Jerzy Kruk | 2013-11-21Analysis perfomed using Arabidopsis thylakoids and total leaf extract, 12% SDS gel, 15 ug protein per lane or 2.5 ug chlorophyll /lane (thylakoids), AB dilution 1:10 000, the product observed at ca. 47 kDa
Accessories
AS03 037 | Clonality: Polyclonal | Host: Rabbit | Reactivity: global antibody and compartment marker for higher plants, lichens, algae, cyanobacteria, dinoflagellates, diatoms
Benefits of using this antibody