1
Anti-mAB-O | Mouse anti-human Abeta protein (3-10) region, oligomer-specific (clone 3E5,F8)
- Product Info
-
Sub class: IgG1, kappa light chain (clone number 3E5, F8) Immunogen: Synthetic peptide chosen from human Abeta (1-42) protein. Host: Mouse Clonality: Monoclonal Purity: Immunogen affinity purified serum in PBS pH 7.4. Format: Lyophilized Quantity: 50 µg Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Dot blot (Dot), ELISA (ELISA), Immunolocalization (IL) Recommended dilution: 10 µg/ml (IL), 1-2 µg/ml (Dot), 1 µg/ml (ELISA capture) Expected | apparent MW: 4.5 kDa - Reactivity
-
Confirmed reactivity: Human Predicted reactivity: Mouse, Bovine, Chicken, Dog, Porcine, Rabbit Not reactive in: No confirmed exceptions from predicted reactivity are currently known - Application Examples
-
Application examples
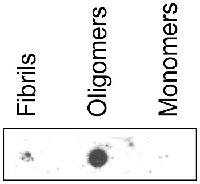
Dot blot
Dot blot reaction of the binding capacity of mAB-O to fibrils, monomers and oligomers. Equal amounts of each sample were spotted on a nitrocellulose membrane and then dried. The membrane was blocked with 5% non-fat milk before incubated for 1 h with anti-mAB-O (25nM) and then with secondary antibody, anti-mouse HRP-conjugated (1:1500). The membrane was washed with PBS containing 0.25% Tween-20 before detection using ECL prime (GE Healthcare).
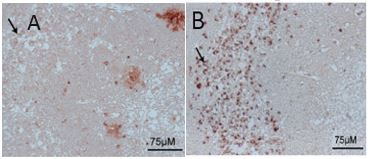
Immunolocalization
IHC used to illustrate the lack of binding of mAB-O to plaques. Tissue sections from the human AD hippocampus were de-waxed and rehydrated in ethanol and then incubated with AS08 357 (A) and mAB-O(B) at RT for 1h. The immunoreactivity was detected with the anti-mouse Peroxidase Reagent Kit (ImmPRESS, Vector Laboratories, Inc.) and then developed using the ImmPACT AEC Peroxidase Substrate kit (Vector Laboratories, Inc.). - Additional Information
-
Additional information (application): Immunolocalization: human tissue was paraffin-embedded and sectioned. De-waxed and rehydrated in an ethanol gradient. Antigens were retrieved in sodium citrate buffer (pH 6) at 95°C for 1 h. The tissue sections were separately incubated for 1 h at RT with primary antibody and antibody binding was visualized with IgG Preoxidase Reagent Kit.
This Monoclonal IgG1, kappa light chain, (clone number 3E5.F8) is specific for human Amyloid-Beta oligomers. - Background
-
Background: Alzheimer's disease (AD) is the most prevalent neurodegenerative disease in the growing population of elderly people. A hallmark of AD is the accumulation of plaques in the brain of AD patients. The plaques predominantly consist of aggregates of amyloid-beta (Abeta), a peptide of 39-42 amino acids generated in vivo by specific, proteolytic cleavage of the amyloid precursor protein. Recent findings however suggest that smaller oligomeric forms of Abeta, formed in parallel to the amyloid plaques, excert the predominant tissue damaging effect.
Specific identification of the oligomeric forms is as a consequence of great interest. Based on a recently published technique a highly oligomer-specific antibody (mAB-O), targeting Abeta oligomers while omitting reactivity towards the monomeric and fibrillar counterpart, has been developed.
- Product Citations
-
Selected references: Brännström et al. (2014). A Generic Method for Design of Oligomer-Specific Antibodies. PLoS ONE. DOI: 10.1371/journal.pone.0090857. - Protocols
-
- Reviews:
-
This product doesn't have any reviews.