1

Anti-Amyloid beta oligomer-specific monoclonal antibody (OMAB)
AS10 932 | Clonality: monoclonal | Host: Mouse | Reactivity: amyloid beta
- Product Info
-
Immunogen: Partly aggregated, recombinant peptide corresponding to the human Abeta (1-40/42). Amino acid sequence: D-A-E-F-R-H-D-S-G-Y-E-V-H-H-Q-K-L-V-F-F-A-E-D-V-G-S-N-K-G-A-I-I-G-L-M-V-G-G-V-V. The epitope is 3-8. The molecular weight of immunogen is 4.5 kDa. Sub class: IgM Host: Mouse Clonality: Monoclonal Purity: Affinity purified in PBS pH 7.4. Format: Lyophilized Quantity: 50 µg Reconstitution: For reconstitution add 100 µl of sterile water Storage: Store lyophilized/reconstituted at 4°C. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: ELISA (ELISA), Immunofluorescence (IF), Immunohistochemistry (IHC) Recommended dilution: Coating antibody at 2 µg/ml (ELISA), 1: 1000 (IF), 1 : 500 (IHC) Expected | apparent MW: 4.5 kDa - Reactivity
-
Confirmed reactivity: Human Abeta oligomers only Predicted reactivity: Rat Not reactive in: No confirmed exceptions from predicted reactivity are currently known - Application Examples
-
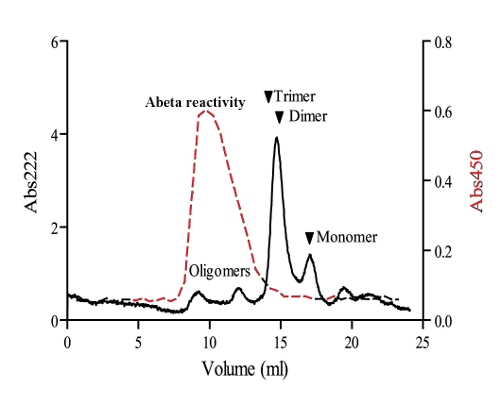
Abeta oligomer-specific antibody was adsorbed to Nunc-Immuno MaxiSorp plates (Nunc, Roskilde, Denmark) at 2 ug/ml in PBS. 1 ml of a 10 uM Aβ(1-42) sample containing a small fraction of Aβ-oligomers was separated using a superdex G75 (10/30) column. Aβ-fractions collected from the SEC were allowed to bind to OMAB plates for 20 minutes at 0°C. All fractions were analyzed and bound Aβwas detected using a polyclonal rabbit anti-Aβ antibody (AS08 328), Agrisera AB, Vännäs, Sweden) at a 1:1000 dilution followed by an anti-rabbit HRP-conjugated secondary antibody at a 1:5000 dilution (GE healthcare). ECBlue (Medicago, Uppsala, Sweden) was used as a substrate for HRP and the signal was detected by measuring the absorbance at 450 nm. Blocking solution and antibody-dilutions were made with 5% Non-fat dry milk in PBST and all washes were performed with PBS containing 0.1% Tween-20 (PBST).
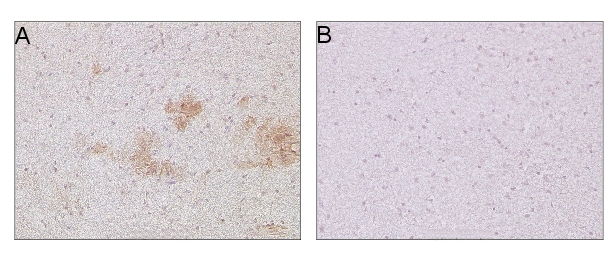
10 µm of coronal sections from fresh-frozen transgenic mouse brain mutant (A) and wild type (B). Post-fixation in 4% formaldehyde solution, 5 min. OMAB antibody diluted 1:500, incubation at 4ºC ON. Mouse on mouse HRP-Polymer kit according to company instructions. Biocare Medical: BC-MM510 (Histolab) DAB substrate kit for peroxidase. Vector Laboratories: SK-4100 (ImmunKemi) Counterstained with Mayers HTX.
- Additional Information
-
Additional information: OMAB antibody has been purified by by ion-exchange chromatography and is supplied in PBS without any additives as carrier proteins or sodium azide.
Binding of OMAB antibody and Abeta oligomers at RT takes about 15 min.
Fibrils are inaccessible for OMAB antibodies therefore if a discrimination between fibrils and oligomers is to be achieved, dot blot can be used. Start with antigen concentration of 500 ng/dot followed by 2X dilution steps. Blocking: non-fat milk and washes with 0.3 % Tween 20 in TBS pH 7.4.Additional information (application): OMAB antibody is a versatile tool within research of Alzheimer’s disease, A sandwich ELISA illustrates its potential regarding its high selectivity towards A beta oligomers.
Sandwich ELISA protocol to detect oligomers
Principle: oligomer specificity is achieved by using an antibody pair/configuration that requires multivalent (divalent or higher) binding. This is only possible using a sandwich-based approach, where the oligomer-specific antibody is used as capture. It cannot be accomplished via direct binding of the oligomers to a surface, as this would not discriminate between oligomers and bound monomers.
The rationale is based on the strong effect of avidity and by using the oligomer-specific antibody as capture, the oligomers (multivalent) are bound strongly via avidity while monomers (monovalent) bind poorly. The potentiating effect can be more than 1000X.
The use of antibodies for detection can be made using either a biotinylated variant of the capture antibody, but frequently also via a polyclonal antibody of another species. Running the assay in a concentration window where oligomer binding is high, but monomer binding remains negligible. This requires an initial optimization through titration of the samples. The design principles are described in Brännström et al. 2014. - Background
-
Background: Soluble oligomeric assemblies of the Amyloid-β peptide are today anticipated to be the direct cause regarding the Alzheimer pathology. As a consequence, oligomeric Aβ-assemblies constitute a very interesting therapeutic target. Identification of Aβ-oligomers is however, technically challenging due to there labile nature and low abundance. Abeta oligomer-specific OMAB antibody is based on the IgM isotype and represents a new concept of Aβ-oligomer binders using a combination of high avidity and very low monovalent affinity. This combination creates a selectivity of the antibody towards the oligomeric fraction and minimizes reactivity towards monomeric species.
- Product Citations
-
Selected references: Li et al. (2026). A Smart Dicyano-Based Fluorescent Probe for In Vivo Amyloid-β Visualization to Illuminate Alzheimer's Disease. Mol Pharm. 2026 Feb 20. doi: 10.1021/acs.molpharmaceut.5c01177.
Wang et al. (2025). Targeting phagocytosis for amyloid-β clearance: implications of morphology remodeling and microglia activation probed by bifunctional chimaeras. Nat Commun. 2025 Aug 30;16(1):8128.doi: 10.1038/s41467-025-63458-3.
Nagashima et al. (2024). Development of Triphenylmethane Dyes for In Vivo Fluorescence Imaging of Aβ Oligomers. ACS Chem Neurosci. 2024 Jun 5;15(11):2233-2242. doi: 10.1021/acschemneuro.4c00053.
Akasanka et al. (2023). In Vivo Near-Infrared Fluorescence Imaging Selective for Soluble Amyloid β Aggregates Using y-Shaped BODIPY Derivative.J Med Chem. 2023 Oct 26;66(20):14029-14046.doi: 10.1021/acs.jmedchem.3c01057.
Pang et al (2021) An App knock-in rat model for Alzheimer's disease exhibiting A? and tau pathologies, neuronal death and cognitive impairments. Cell Res. 2021 Nov 17. doi: 10.1038/s41422-021-00582-x. Epub ahead of print. PMID: 34789895.
Oh et al. (2020). Associative Interactions among Zinc, Apolipoprotein E, and Amyloid-? in the Amyloid Pathology. Int J Mol Sci. 2020 Jan 25;21(3). pii: E802. doi: 10.3390/ijms21030802.
Henning-Knechtel et al. (2020). Designed Cell-Penetrating Peptide Inhibitors of Amyloid-beta Aggregation and Cytotoxicity. Cell Reports Physical Science,Volume 1, Issue 2, 26
Zhang et al. (2019). Brains of rhesus monkeys display A? deposits and glial pathology while lacking A? dimers and other Alzheimer's pathologies. Aging Cell. 2019 Jun 4:e12978. doi: 10.1111/acel.12978.
Kumar et al. (2018). Peptidomimetic-Based Multidomain Targeting Offers Critical Evaluation of A? Structure and Toxic Function. J Am Chem Soc. 2018 May 30;140(21):6562-6574. doi: 10.1021/jacs.7b13401.
Zhao et al. (2016). Antiamyloidogenic Activity of ABeta42-Binding Peptoid in Modulating Amyloid Oligomerization. Small. 2016 Oct 7. doi: 10.1002/smll.201602857.
Richman et al. (2013). In Vitro and Mechanistic Studies of an Anti-Amyloidogenic Self-Assembled Cyclic D,L-#-Peptide Architecture. J. Americal Chemical Societ, Jan 19.
Lindhagen-Persson et al. (2010). Amyloid-Beta Oligomer Specificity Mediated by the IgM Isotype – Implications for a Specific Protective Mechanism Exerted by Endogenous Auto-Antibodies. PLoS ONE. - Protocols
- Antibody protocols
- Reviews:
-
This product doesn't have any reviews.