1
Anti-VDAC1-5 | Voltage-dependent anion-selective channel protein 1-5
AS07 212 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A. thaliana, A. palmeri, S. oleracea, di and monocots | Cellular [compartment marker] of mitochondrial outer membrane
- Product Info
-
Immunogen: KLH-conjugated peptide conserved in all known higher plant VDAC proteins including Arabidopsis thaliana VDAC1 UniProt: Q9SRH5 , TAIR: AT3G01280, VDAC2 UniProt F4K3R8-1 , TAIR: AT5G67500, VDAC3 UniProt: Q9SMX3-1, TAIR: AT5G15090, VDAC4 UniProt: Q9FKM2-1, TAIR:AT5G57490, VDAC5 UniProt: Q9M2W6-1, TAIR: AT3G49920
Host: Rabbit Clonality: Polyclonal Purity: Immunogen affinity purified serum in PBS pH 7.4. Format: Lyophilized Quantity: 50 µg Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: 2D Blue Native PAGE (2D BN-PAGE), Western blot (WB) Recommended dilution: 1 : 500 (IL), 1 : 5000, 2-30 µg protein/lane (WB) Expected | apparent MW: 29 kDa (for Arabidopsis thaliana)
- Reactivity
-
Confirmed reactivity: Arabidopsis thaliana, Amaranthus palmeri, Beta vulgaris, Brassica oleracea var. botrytis, Brassica rapa subsp. rapa, Citrus sinensis, Fortunella margarita Swingle, Oryza sativa, Papaver sp. pollen tubes (IL), Spinacia oleracea, Physcomitrium patens, Solanum lycopersicum, Zea mays Predicted reactivity: Arabidopsis alpina, Aundo donax, Brachypodium distachyon, Brassica campestris, Brassica napus, Brassica rapa subsp. pekinensis, Capsella rubella, Citrus clementina, Eutrema salsugineum, Glycine max, Glycine soja, Gossypium arboreum, Hoedum vulgare var. distichum, Jatropha curcas, Medicago truncatula, Mesembryanthemum crystallinum, Morus notabilis, Nicotiana tabacum, Phaseolus coccineus, Phaseolus vulgaris, Pisum sativum, Plantago major, Prunus persica, Ricinus communis, Solanum tuberosum, Sorghum bicolor, Theobroma cacao, Triticum aestivum, Vitis vinifera
Species of your interest not listed? Contact usNot reactive in: Chlamydomonas reinhardtii, Glycine max, diatoms, Malus domestica, Saccharomyces cerevisiae
- Application Examples
-
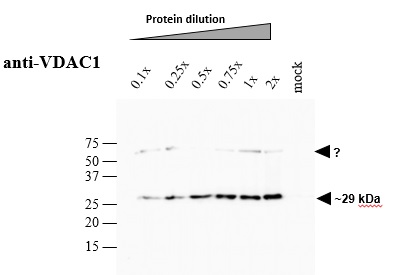
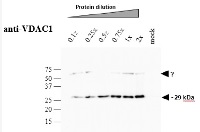
Crude membrane proteins were separated on 12% SDS-PAGE and blotted 1h to PVDF. Blots were blocked immediately following transfer in 5% blocking reagent (BioRad, 170-6404) in 50 mM Tris, 150 mM sodium chloride pH 7.5 with 0.1% (v/v) Tween-20 (TBS-T) for 1h at room temperature with agitation. Blots were incubated in the primary antibody in 1: 5000 dilution for over-night at 4°C with agitation. The antibody solution was decanted and the blot was rinsed briefly twice, then washed 3 times for 5 min in TBS-T at room temperature with agitation. Blots were incubated in secondary antibody (Goat anti-rabbit IgG horse radish peroxidase conjugated) diluted to 1:5000 in 0.2% blocking solution for 1h at room temperature with agitation. The blots were washed as above and developed for 1~2 min with chemiluminescent detection reagent, according the manufacturers instructions. Images of the blots were obtained using a CCD imager (LAS4000 GE) and by ImageQuant software (GE).Arabidopsis thaliana membrane extraction and SDS–PAGE analysis About 200 mg (gFW) Arabidopsis seedlings (3-week-old), grown on 1% MS-agar plates, was ground with mortar and pestle in the presence of 2 ml extraction buffer [75 mM MOPS-KOH, 0.6 M Sucrose, 4 mM EDTA, 0.2% PVP-40, 0.2% BSA, 8 mM L-cystein, pH 7.6] and the protease inhibitor cocktail ‘complete Mini’ from Roche Diagnostics GmbH (Mannheim, Germany). Crude membrane extracts were prepared essentially as described in Colas des Francs-Small et al. (2012). The membranous fraction was obtained by centrifugation at 22,000 g for 10 min at 4oC. The pellet containing the crude membranous fraction was washed twice with wash buffer [37.5 mM MOPS-KOH, 0.3 M Sucrose, 2 mM EDTA pH 7.6]. The samples were kept frozen at -80oC until used. For SDS-PAGE, an aliquot equivalent to 10 mg (i.e. 1x dilution) of crude Arabidopsis membrane extracts was solubilized in 3x Laemmli sample buffer (Bio-Rad) and the proteins were analyzed by SDS-gel electrophoresisCourtesy of Dr. Oren Ostersetzer, The Hebrew University of Jerusalem, Israel.jpg)
Fixation and Immunolocalization
(A) full confocal stacks; (B) Single confocal section
Pollen tubes were fixed in 400 μM 3-maleimodobenzoic acid N-hydroxysuccinimide ester (MBS, Pierce) for 6 min at 20ºC, followed by 2% formaldehyde (1 h, 4°C). Cells were washed three times in 1x TBS then once in MES buffer (15 mM MES, pH 5.0), then incubated in 0.05% cellulose/0.05% macerozyme with 0.1% Triton X-100 in MES buffer containing 0.1 mM PMSF and 1 % BSA for 15 min. Cells were washed once in MES, then twice in TBS and then incubated in blocking solution (1% BSA in TBS) for 30 min at room temperature. Pollen was incubated with anti-VDAC1 antibodyies diluted in blocking solution (at 1:500) overnight at 4°C. Following TBS washes pollen was then incubated with the secondary antibody for 1.5 h at room temperature followed by further TBS washes. Pollen tubes were mounted on slides with 5 μL of Vectashield + DAPI (Vector Laboratories, USA) and coverslips sealed with nail varnish.Method taken from Poulter et al (submitted) Actin-binding proteins implicated in formation of the punctate actin foci stimulated by the self-incompatibility response in Papaver . Submitted to Plant Physiology.
Courtesy Professor Noni Franklin-Tong, University of Birmingham, UK
Application examples: 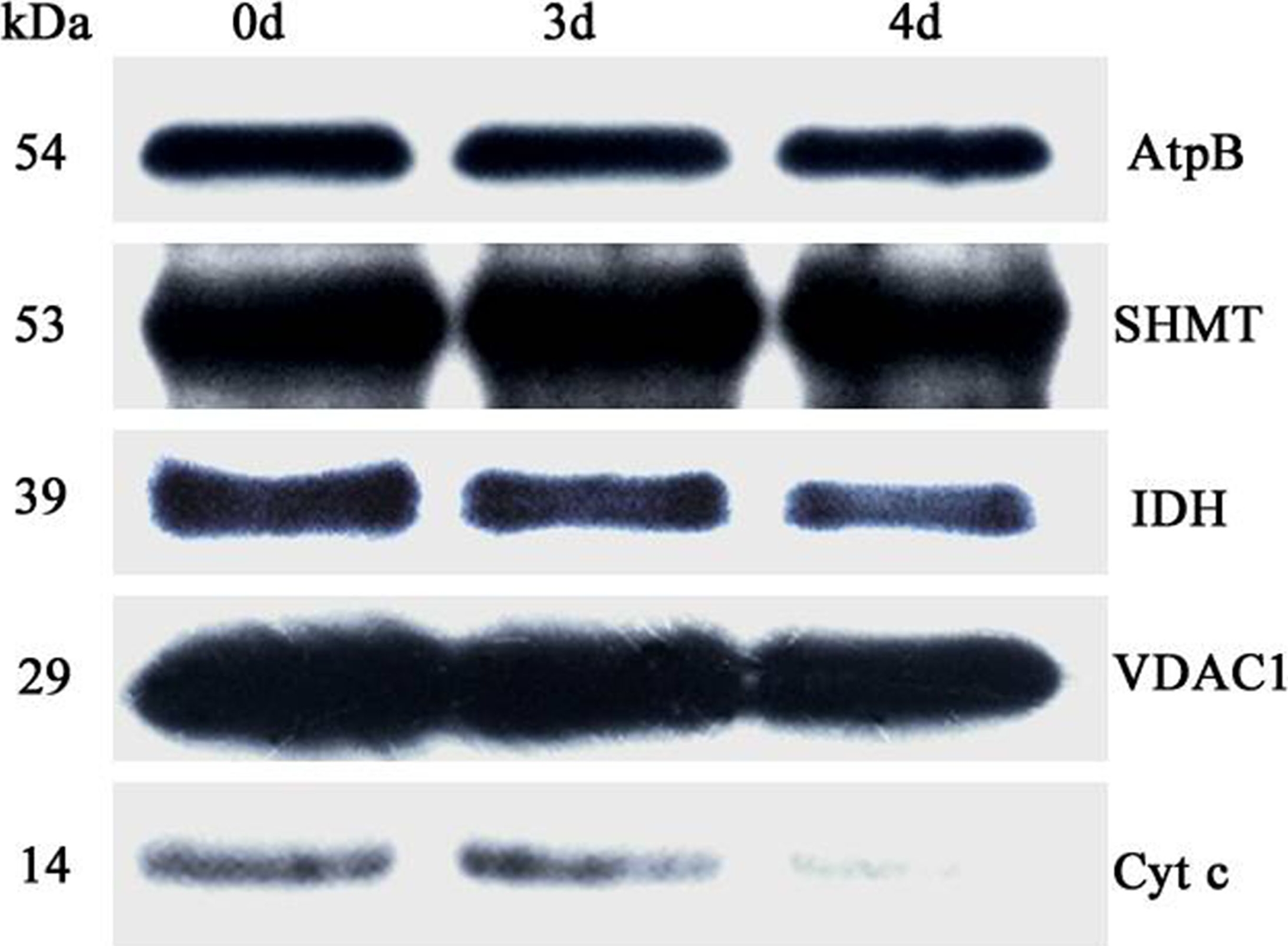
Reactant: Oryza sativa (Asian rice)
Application: Western Blotting
Pudmed ID: 27124767
Journal: PLoS One
Figure Number: 6A
Published Date: 2016-04-29
First Author: Yin, G., Whelan, J., et al.
Impact Factor: 2.942
Open PublicationAbundance of a variety of known mitochondrial proteins by the immunoblot analysis of mitochondria isolated from 0 d, 3 d, and 4 d aged rice embryos after 48 h imbibition.Total 10 ?g protein was separated by SDS gel electrophoresis and blotted to supported polyvinylidene difluoride, then probed with antibodies against beta subunit of ATP synthase (AtpB), serine hydroxymethyltransferase (SHMT), isocitrate dehydrogenase (IDH), voltage dependent anion channel 1 (VDAC1) and cytochrome c (Cyt c).
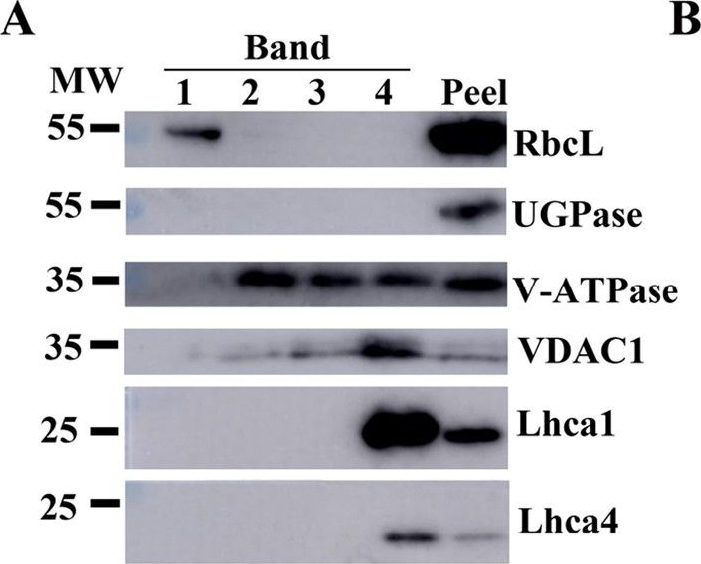
Reactant: Plant
Application: Western Blotting
Pudmed ID: 29423236
Journal: Hortic Res
Figure Number: 3A
Published Date: 2018-02-10
First Author: Zhu, M., Lin, J., et al.
Impact Factor: 6.072
Open PublicationAssessment of the purity of isolated elaioplasts using immunoblots.Different fractions of the sucrose gradient (Band 1 to Band 4) are compared to peel proteins using antibodies for plastid stroma large Rubisco subunit (RbcL), cytosolic UGPase, vacuolar (v)-ATPase, mitochondrial voltage-dependent anion-selective channel protein 1 (VDAC1), and photosynthesis Light-harvesting complex (Lhca1 and Lhca4); b Coomassie blue protein profiles of purified elaioplasts (Band 1 to Band 4) from kumquat peel, as compared with purified chromoplast (Chro.) from sweet orange flesh and total kumquat peel proteins
- Additional Information
-
Additional information: Cellular [compartment marker] of mitochondrial outer membrane for western blot, Additional information (application): Amount of mitochondrial fraction detected by anti-VDAC1 antibody was from 2-10 µg.Immunolocalization method description and images are available hereBlue-native (2D BN/SDS-PAGE) methodology is described in Piechota et al. 2010 - Background
-
Background: VDAC proteins are porin-type, beta-barrel diffusion pores. Prominently localized in the outer mitochondrial membrane and involved in metabolite exchange.
- Product Citations
-
Selected references: Holzner et al. (2026). The chloroplast ionome shines light on the dynamics of organellar iron homeostasis. Plant Cell. 2026 Jan 29:koag017. doi: 10.1093/plcell/koag017.
Iglesias-Sanchez et al. (2025).Activation of Alternative Oxidase Ensures Carbon Supply for Ethylene and Carotenoid Biosynthesis During Tomato Fruit Ripening. Plant Physiol. 2025 Oct 31;199(3):kiaf516. doi: 10.1093/plphys/kiaf516.
Konishi and Noguchi (2025). The Mitochondrial Respiratory Chain is Important as an Electron Sink to Avoid Over-Reduction of Chloroplasts in Arabidopsis thaliana Leaves under High-Light Stress. Plant Cell Physiol. 2025 Jun 5:pcaf062. doi: 10.1093/pcp/pcaf062.
Boussardon et al. (2025). The atypical proteome of mitochondria from mature pollen grains. Curr Biol . 2025 Jan 21:S0960-9822(24)01705-6. doi: 10.1016/j.cub.2024.12.037.
Soria et al. (2024).Functional resilience: An active oxidative phosphorylation system prevails amid foreign proteins in holoparasitic plants. Current Plant Biology Volume 37, March 2024, 100322.
Wittmann at al. (2024). Dual plastid targeting of Protoporphyrinogen Oxidase 2 in Amaranthaceae promotes herbicide tolerance. Plant Physiol. 2024 Feb 8:kiae062.doi: 10.1093/plphys/kiae062.
Bao et al. (2023). Aberrant accumulation of ceramides in mitochondria trigger cell death requiring autophagy in Arabidopsis. J Exp Bot . 2023 Dec 9:erad456.doi: 10.1093/jxb/erad456.
Xue et al.(2023). The PtdIns3P phosphatase MtMP promotes symbiotic nitrogen fixation via mitophagy in Medicago truncatula. iScience. 2023 Sep 15;26(10):107752.doi: 10.1016/j.isci.2023.107752.
Belykh et al. (2022) Responses of genes of DNA repair, alternative oxidase, and pro-/antioxidant state in Arabidopsis thaliana with altered expression of AOX1a to gamma irradiation. Int J Radiat Biol. 2022;98(1):60-68. doi: 10.1080/09553002.2022.1998712. Epub 2021 Nov 11. PMID: 34714725.
Li et al. (2021) Isolation and comparative proteomic analysis of mitochondria from the pulp of ripening citrus fruit. Hortic Res. 2021 Feb 1;8(1):31. doi: 10.1038/s41438-021-00470-w. PMID: 33518707; PMCID: PMC7848011.
Tarasenko et al. (2020). Plant mitochondrial subfractions have different ability to import DNA. Theor. Exp. Plant Physiol. doi.org/10.1007/s40626-020-00167-w
Garmash et al. (2020). Altered levels of AOX1a expression result in changes in metabolic pathways in Arabidopsis thaliana plants acclimated to low dose rates of ultraviolet B radiation. Plant Sci. 2020 Feb;291:110332. doi: 10.1016/j.plantsci.2019.110332.
Bai et al. (2019). Overexpression of soybean GmPLD? enhances seed oil content and modulates fatty acid composition in transgenic Arabidopsis. Plant Science Volume 290, January 2020, 110298.
Klinger et al. (2019). The signal distinguishing between targeting of outer membrane Beta-barrel protein to plastids and mitochondria in plants. Biochim Biophys Acta Mol Cell Res. 2019 Jan 8;1866(4):663-672. doi: 10.1016/j.bbamcr.2019.01.004.
Zhu et al. (2018). A comprehensive proteomic analysis of elaioplasts from citrus fruits reveals insights into elaioplast biogenesis and function. Hortic Res. 2018 Feb 7;5:6. doi: 10.1038/s41438-017-0014-x.
Kang et al. (2018). Autophagy-related (ATG) 11, ATG9 and the phosphatidylinositol 3-kinase control ATG2-mediated formation of autophagosomes in Arabidopsis. Plant Cell Rep. 2018 Jan 19. doi: 10.1007/s00299-018-2258-9.
Wang and Auwerx (2017). Systems Phytohormone Responses to Mitochondrial Proteotoxic Stress. Mol Cell. 2017 Nov 2;68(3):540-551.e5. doi: 10.1016/j.molcel.2017.10.006.
Yin et al. (2016). Comprehensive Mitochondrial Metabolic Shift during the Critical Node of Seed Ageing in Rice. PLoS One. 2016 Apr 28;11(4):e0148013. doi: 10.1371/journal.pone.0148013. eCollection 2016.
de Michele et al. (2016). Free-Flow Electrophoresis of Plasma Membrane Vesicles Enriched by Two-Phase Partitioning Enhances the Quality of the Proteome from Arabidopsis Seedlings. J Proteome Res. 2016 Mar 4;15(3):900-13. doi: 10.1021/acs.jproteome.5b00876. Epub 2016 Feb 4.
Li et al. (2015). A Chaperone Function of NO CATALASE ACTIVITY1 Is Required to Maintain Catalase Activity and for Multiple Stress Responses in Arabidopsis. Plant Cell. 2015 Feb 19. pii: tpc.114.135095.
Rurek et al. (2015). Biogenesis of mitochondria in cauliflower (Brassica oleracea var. botrytis) curds subjected to temperature stress and recovery involves regulation of the complexome, respiratory chain activity, organellar translation and ultrastructure. Biochim Biophys Acta. 2015 Jan 21. pii: S0005-2728(15)00016-X. doi: 10.1016/j.bbabio.2015.01.005.
Armbruster et al. (2014). Ion antiport accelerates photosynthetic acclimation in fluctuating light environments. Nat Commun. 2014 Nov 13;5:5439. doi: 10.1038/ncomms6439
Hsueh et al. (2014). The chloroplast outer envelope protein P39 in Arabidopsis thaliana belongs to the Omp85 protein family. Proteins. 2014 Nov 17. doi: 10.1002/prot.24725.
Takahashi et al. (2014). Transport of rice cyclobutane pyrimidine dimer (CPD) photolyase into mitochondria relies on a targeting sequence located in its C-terminal internal region.
Alcantar-Aguirre et al.(2013).ATP produced by oxidative phosphorylation is channeled toward hexokinase bound to mitochondrial porin (VDAC) in beetroots (Beta vulgaris). Planta, March 17.
Lang et al. (2011).Simultaneous isolation of pure and intact chloroplasts and mitochondria from moss as the basis for sub-cellular proteomics. Plant Cell Rep. 2011 Feb;30(2):205-15.doi: 10.1007/s00299-010-0935-4. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts - Reviews:
-
Jane Lee | 2023-12-07This antibody works very well for our samples so we are very satisfied with thisPeter Ma | 2019-01-09This antibody works very well for our samples. The membrane proteins from the Arabidopsis roots are subjected to 10% SDS-PAGE and then detected with this antibody (1:5000). The results shows that this antibody is quite specific and sensitive.Michal RUREK | 2014-02-1415 µg of mitochondrial proteins from cauliflower (Brassica oleracea var. botrytis) curds (apical layer) was separated on 12 % SDS-PAGE (Laemmli- type) and blotted 1h to Immobilone P (Millipore) using Sedryt apparatus (Kucharczyk). After blocking (5% milk in PBS-T) for 1 h at RT, blots were incubated in the primary antibody at a dilution of 1/1000 (could be shifted down to 1/10000) for overnight at 4 deg. with agitation. The primary antibody, diluted in 2% milk in PBS-T was reused several times. Secondary, anti-rabbit HRP- linked antibodies were bound at 1/10000 dilution at RT (1h). Single band of ca. 30 kDa was detected using standart GE Healthcare ECL reagents.David Macherel | 2009-07-23The antibody yields good signal on western blots with extracts from germinating Arabidopsis seeds and young seedlings. We use it at 1/1000 dilution followed by ECL detectionEmanuel Schmid | 2009-03-05We did first attempts in our lab with the previous version of this antibody. It worked fine for enriched Mitochondria samples but unfortunately not in crude Arabidopsis samples. This new version of the VDAC1 antibody solves completely the previous problems, We were now able to detect the VDAC1 even in crude Arabidopsis samples.-------------------------------------------Materials and Methods:Crude Arabidopsis extract was obtained by blending leaves and filtering it through Miracloth. 7 microgramm protein of this mix was used for the SDS-Page.Blocking overnight @ 4 °C in 5% milk in PBS. Antibody incubation for 3h @ RT, 1:1000 in PBS 5% milk. Detection with Protein A.Bronwyn Barkla | 2008-11-17We are very satisfied with this antibody.
Accessories

AS07 212-HRP | Clonality: Polyclonal | Host: Rabbit | Reactivity: A.thaliana, di and monocots | Cellular [compartment marker] of mitochondrial outer membrane


