| Question: Why are we recommended to use urea for the detection of some proteins? Answer: Following a Western blot protocol with SDS, and conducting it in denatured conditions, some proteins may actually patrially re-fold, which is going to make some antibody binding sites not accessible. To keep the protein unfolded, and the epitopes for antibody binding accessible, 6M urea must sometimes be included in the extraction and loading buffers. Some laboratories are using urea in their standard protocol. This will however not work, if the antibody is binding to discontinued epitopes, found only in the folded protein. Proteins can also be fixed and unfolded on a membrane following the transfer, by drying up the PVDF membrane, protein side up. Including urea and drying the PVDF membrane following the transfer, will help to keep the target protein unfolded, and make antibody binding possible. | 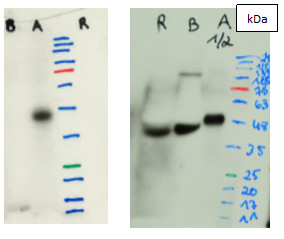 Anti-VDE antibodies were used on extracts from Brachypodium distachyon, Arabidopsis thaliana and Oryza sativa. Adjustment of the buffer to 7M Urea, 2M Thiourea, 4% CHAPS, 35 mM Tris pH 8, resulted in deteciton of VDE in not only Arabidopsis thaliana, but two more species, namely Brachypodium distachyon and Oryza sativa. |
Latest
Will the precipitation of SDS in the stripping buffer affect antibody stripping efficiency?2025-04-29 How to check if protein transfer from a gel to a membrane was efficient
2025-04-15 How to detect membrane proteins on Western blot?
2025-03-27 Rinse Right: The Hidden Power of Washing in Western Blots
2025-02-11 Can one Western blot protocol fit all antibodies?
2025-02-07 How to reconstitute lyophilized antibodies?
2025-01-17 What information to look for before purchasing an antibody?
2024-12-30 Membrane activation before protein transfer: which one to use, methanol or ethanol?
2024-12-23 Comparing antibodies between each other, why does it not work?
2024-11-25 How to remove cross-reactivity to Rubisco of a primary antibody?
2024-09-24
Archive
- April - 2025
- March - 2025
- February - 2025
- January - 2025
- December - 2024
- November - 2024
- September - 2024
- July - 2024
- June - 2024
- May - 2024
- March - 2024
- February - 2024
- December - 2023
- November - 2023
- September - 2023
- July - 2023
- May - 2023
- March - 2023
- January - 2023
- December - 2022
- November - 2022
- October - 2022
- September - 2022
- August - 2022
- June - 2022
- May - 2022
- March - 2022
- February - 2022
- January - 2022
- November - 2021
- October - 2021
- August - 2021
- June - 2021
- May - 2021
- April - 2021
- March - 2021
- February - 2021
- January - 2021
- December - 2020
- November - 2020
- October - 2020
- September - 2020
- August - 2020
- July - 2020
- June - 2020
- May - 2020
- April - 2020
- March - 2020
- January - 2020
- November - 2019
- October - 2019
- March - 2019
- April - 2017
- February - 2017
- May - 2016
- February - 2014
- September - 2013
- December - 2010
