1
Anti-LHCSR1
AS14 2819 | Clonality: Polyclonal | Host: Rabbit | Reactivity: Bryopsis corticulans, Chlamydomonas reinhardtii, Nannochloropsis gaditana
- Data sheet
- Product Info
-
Immunogen: KLH-conjugated synthetic peptide derived from LHCSR1 protein sequence from Chlamydomonas reinhardtii, UniProt: P93664
Host: Rabbit Clonality: Polyclonal Purity: Immunogen affinity purified serum in PBS pH 7.4. Format: Lyophilized Quantity: 50 µg Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Western blot (WB) Recommended dilution: 1 : 1000 (WB) Expected | apparent MW: 27 kDa
- Reactivity
-
Confirmed reactivity: Bryopsis corticulans, Chlamydomonas reinhardtii, Nannochloropsis gaditana
Predicted reactivity:
Species of your interest not listed? Contact usNot reactive in: Lobosphaera incisa, Phaeodactylum tricornutum - Application Examples
- Application example
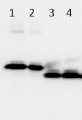
10 ug of a total cell extract of Chlamydomonas reinhardtii: WT _P _HL_High CO2 (1), WT_P_HL (2), Npq4_P _HL (3), were separated on Bolt® 4-12% Bis-Tris Plus Gels (precast) and blotted to PVDF using Bolt® transfer system for 1h . Blots were blocked with 5% BSA/milk for 1h at room temperature (RT) with agitation. Blot was incubated in the primary antibody at a dilution of 1: 1 000 for 1h at RT with agitation. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 15 min and3 times for 5 min in PBS-T at RT with agitation. Blot was incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, AS09 602, Agrisera) diluted to 1:10 000 in for 1h at RT withagitation. The blot was washed as above and developed for 5 min with ECL according to the manufacturer's instructions. Exposure time was 10 seconds.P: photoautotrophically grown cellsHL: High light illumination(light intensity:500 μE)
Courtesy Dr. Roberta Croce, Biophysics of Photosynthesis Dep. Physics and Astronomy Faculty of Sciences VU University Amsterdam, The NetherlandsApplication examples: 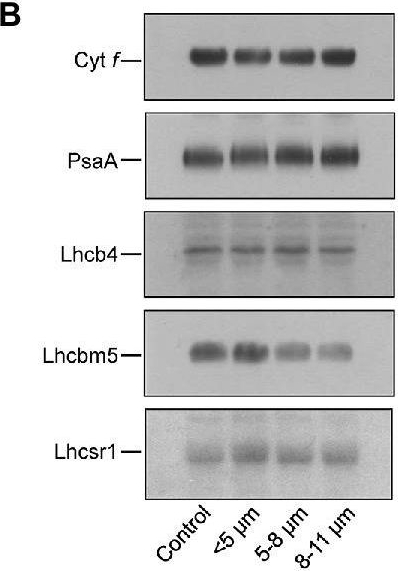
Reactant: Chlamydomonas reinhardtii (Green Alga)
Application: Western Blotting
Pudmed ID: 36119589
Journal: Front Plant Sci
Figure Number: 10B
Published Date: 2022-09-20
First Author: Szyszka-Mroz, B.
Impact Factor: 6.308
Open PublicationCoomassie stained SDS-PAGE analysis of thylakoid membrane proteins from control and different-sized cell fractions of C. priscuii(A). Lanes of SDS-PAGE were loaded on an equal Chl basis. Values on the left indicate apparent molecular mass (kDa). B – Representative Western blots (B) and densitometric analysis (C) of polypeptides probed with antibodies raised against Cyt f, Lhcbm5, Lhcb4, and PsaA polypeptides in thylakoid membranes isolated from different-sized cell fractions of C. priscuii. Mean values ± SE were calculated from 3 independent experiments. The presented data were normalized to the relative abundance of the respective polypeptides in the control whole cell fraction.
- Additional Information
-
Additional information (application): This antibody is also recognizing recombinant LHCSR1 overexpressed in E.coli as described in Perozeni et al. (2020). - Background
-
Background: LHCSR1 (Stress-related chlorophyll a/b binding protein 1) plays a role in an efficient enery dissipation process, called non-photochemical quenching (NPQ), in Chlamydomonas reinhardtii.
- Product Citations
-
Selected references: Arend et al. (2023) Widening the landscape of transcriptional regulation of green algal photoprotection. Nat Commun. 2023 May 10;14(1):2687. doi: 10.1038/s41467-023-38183-4. PMID: 37164999.
Ruiz-Sola et al. (2023) Light-independent regulation of algal photoprotection by CO2 availability. Nat Commun. 2023 Apr 8;14(1):1977. doi: 10.1038/s41467-023-37800-6.
McQuilian et al. (2023). Proteomic characterization of a lutein-hyperaccumulating Chlamydomonas reinhardtii mutant reveals photoprotection-related factors as targets for increasing cellular carotenoid content. Biotechnol Biofuels Bioprod . 2023 Nov 4;16(1):166. doi: 10.1186/s13068-023-02421-0.
Cazzaniga et al. (2022). Engineering astaxanthin accumulation reduces photoinhibition and increases biomass productivity under high light in Chlamydomonas reinhardtii. Biotechnol Biofuels Bioprod. 2022 Jul 11;15(1):77. doi: 10.1186/s13068-022-02173-3. PMID: 35820961; PMCID: PMC9277849.
Redekop et al. (2020). PsbS Contributes to Photoprotection in Chlamydomonas Reinhardtii Independently of Energy Dissipation . Biochim Biophys Acta Bioenerg . 2020 Jun 1;1861(5-6):148183.doi: 10.1016
Lammermann et al. (2020). Ubiquitin ligase component LRS1 and transcription factor CrHy5 act as a light switch for photoprotection in Chlamydomonas. doi.org/10.1101/2020.02.10.942334 bioRxiv
Roach et al. (2020). The non-photochemical quenching protein LHCSR3 prevents oxygen-dependent photoinhibition in Chlamydomonas reinhardtii. J Exp Bot. 2020 Jan 16. pii: eraa022. doi: 10.1093/jxb/eraa022.
Gabilly et al. (2019). Regulation of photoprotection gene expression in Chlamydomonas by a putative E3 ubiquitin ligase complex and a homolog of CONSTANS. Proc Natl Acad Sci U S A. 2019 Aug 12. pii: 201821689. doi: 10.1073/pnas.1821689116.
Tian et al. (2019). pH dependence, kinetics and light-harvesting regulation of nonphotochemical quenching in Chlamydomonas. Proc Natl Acad Sci U S A. 2019 Apr 23;116(17):8320-8325. doi: 10.1073/pnas.
Aihara et al. (2019). Algal photoprotection is regulated by the E3 ligase CUL4-DDB1DET1. Nat Plants. 2019 Jan;5(1):34-40. doi: 10.1038/s41477-018-0332-5.
Kosuge et al.(2018). LHCSR1-dependent fluorescence quenching is mediated by excitation energy transfer from LHCII to photosystem I in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 2018 Apr 3;115(14):3722-3727. doi: 10.1073/pnas.1720574115.
Giovagnetti et al. (2018). A siphonous morphology affects light-harvesting modulation in the intertidal green macroalga Bryopsis corticulans (Ulvophyceae). Planta. 2018 Feb 19. doi: 10.1007/s00425-018-2854-5.
Chukhutsina et al. (2017). Photoprotection strategies of the alga Nannochloropsis gaditana. Biochim Biophys Acta. 2017 Jul;1858(7):544-552. doi: 10.1016/j.bbabio.2017.05.003.
Allorent et al. (2016). UV-B photoreceptor-mediated protection of the photosynthetic machinery in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 2016 Dec 20;113(51):14864-14869. doi: 10.1073/pnas.1607695114. Epub 2016 Dec 5.
Dinc et al. (2016). LHCSR1 induces a fast and reversible pH-dependent fluorescence quenching in LHCII in Chlamydomonas reinhardtii cells. Proc Natl Acad Sci U S A. 2016 Jul 5;113(27):7673-8. doi: 10.1073/pnas.1605380113. Epub 2016 Jun 22.
Correa-Galvis et al. (2016). Photosystem II Subunit PsbS Is Involved in the Induction of LHCSR Protein-dependent Energy Dissipation in Chlamydomonas reinhardtii. J Biol Chem. 2016 Aug 12;291(33):17478-87. doi: 10.1074/jbc.M116.737312. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts
Agrisera Educational Posters Collection - Reviews:
-
Anxhela | 2020-10-21Antibody works relatively good, but is a bit expensive for the amount (considering the high concentration that you need for a single Western Blot).