1
Anti-RbcL | Rubisco large subunit, form I (affinity purified)
AS03 037A | Clonality: Polyclonal | Host: Rabbit | Reactivity: [global antibody] for higher plants, lichens, algae, cyanobacteria, dinoflagellates, diatoms compartment marker of chloroplast stroma in higher plants and cytoplasm in cyanobacteria
Benefits of using this antibody
- Product Info
-
Immunogen: KLH-conjugated synthetic peptide conserved across all known plant, algal and (cyano)bacterial RbcL protein sequences (form I L8S8 and form II L2), including Arabidopsis thaliana O03042, Hordeum vulgare P05698, Oryza sativa P0C510, Chlamydomonas reinhardtii P00877, Synechococcus PCC 7920 A5CKC5
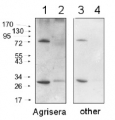
Host: Rabbit Clonality: Polyclonal Purity: Immunogen affinity purified serum in PBS pH 7.4. Format: Lyophilized Quantity: 50 µg Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Immunofluorescence (IF), Western blot (WB) Recommended dilution: 1 : 5000-10 000 (WB) Expected | apparent MW: 52.7 kDa (Arabidopsis thaliana), 52.5 kDa (cyanobacteria), 52.3 (Chlamydomonas reinhardtii)
- Reactivity
-
Confirmed reactivity: Agostis stolonifera cv. Penncross, Arabidopsis thaliana, Apium graveolens, Artemisia annua, Atrichum undulatum, Attheya longicornis, Baculogypsina sphaerulata (benthic foraminifer), Beta vulgaris, Begonia sp., Bienertia sinuspersici, Brassica napus, Kandelia candel, Camellia sinensis, Cannabis sativa L., Chaetoceros furcellatus, Chlorococcum dorsiventrale, Colobanthus quitensis, Cicer arietinum, Chenopodium quinoa, Chlamydomonas raudensis, Chlamydomonas reinhardtii, Colobanthus quitensis Kunt Bartl, Chlorella sorokiniana, Chlorella vulgaris, Coscinodiscus concinnus, Cyanophora paradoxa, Cylindrospermopsis raciborskii CS-505, Cynara cardunculus, Emiliana huxleyi, Euglena gracilis, Ficus carica, Fortunella margarita Swingle, Fraxinus mandshurica, Fucus vesiculosus, Gladieria sulphuraria, Glycine max, Gonyaulax polyedra, Gongolaria barbata, Guzmania hybrid, Heterosigma akashiwo, Hevea, Hordeum vulgare, Hypnum cupressiforme, Jatropha curcas, Karenia brevis (C.C.Davis) s) G.Hansen & Ø.Moestrup (Wilson isolate), Kochia prostrata, Lathyrus sativus, Liquidambar formosana, Malus domestica, Medicago truncatula, Micromonas pusila, Nicotiana benthamiana, Nicotiana tabacum, Nostoc punctiforme PCC 73102, Oryza sativa, Panicum virgatum, Petunia hybrida cv. Mitchell, Phaeodactylum tricornutum, Physcomitrium patens, Pisum sativum, olytrichum formosum, Porosira glacialis,, Porphyra sp., Ricinus communis, Robinia pseudoacacia, Rhytidiadelphus squarrosus, Saccharum sp., Schima superba, Skeletonema costatum (diatom), Skeletonema marinoi (diatom), Solanum lycopersicum, Spinacia oleracea, lichens, Stanleya pinnata, Stevia rebaudiana, Symbiodinium sp., Synechococcus PCC 7942, Synechococcus elongatus UTEX 2973, Rhoeo discolor, Thalassiosira pseudonana, Thermosynechococcus elongatus, Triticum aestivum, Prochlorococcus sp. (surface and deep water ecotype), Triticum aestivum, dinoflagellate endosymbionts (genus Symbiodinium), extreme acidophilic verrucomicrobial methanotroph Methylacidiphilum fumariolicum strain SolV, Thalassiosira punctigera, Tisochrysis lutea, Verbascum lychnitis, Vitis vinifera, Quercus ilex Predicted reactivity: Alpha proteobacteria, Algae (brown and red) including Galdieria sulphuraria, Arthrospira platensis, Dicots, Benincasa hispida, Kalanchoe fedtschenkoi; Beta-proteobacteria, Conifers, Cryptomonads, Cyanobacteria (prochlorophytes), Eragrostis tef, Gamma-proeobacteria, Liverworts, Manihot esculenta, Marchantia polymorpha, Miscanthus giganteus, Monocots, Mosses, Suaeda glauca, Welwitschia; Nannochloropsis sp., Picochlorum sp., Porphyridium purpureum, Zea mays, Zosteria marina
For detection in Rhodospirillaceae use product AS15 2955
Species of your interest not listed? Contact usNot reactive in: No confirmed exceptions from predicted reactivity are currently known - Application Examples
-
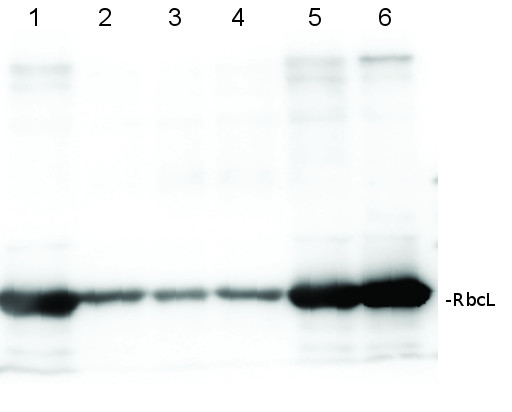
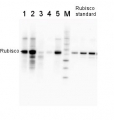
Total protein from Populus T89 were extracted with “KEB buffer”, precipitated with ethanol on ice and denatured with “loading buffer” at 100°C for 10 min, separated on 8% SDS-PAGE and blotted O/N to PVDF using (wet blot) tank transfer. Blots were blocked with 5%TBS milk, for 1h at room temperature (RT) with agitation. Blot was incubated in the primary antibody at a dilution of 1: 1 000 TBS for 2h at RT with agitation. The antibody solution was decanted and the blot was rinsed briefly with TBS-T, then washed for 1h in TBS-T at RT with agitation. Blot was incubated in secondary antibody (goat anti-rabbit IgG HRP-conjugated, from Agrisera, AS09 602) diluted to 1:5000 in TBS-M (milk 5%) for 1h at RT with agitation. The blot was washed as above and developed with chemiluminescent detection reagent, for 10s increment until exposure time of 30s total.
Courtesy Dr. Mark Ruhl, Umeå Plant Science Centre, Sweden - Additional Information
-
Additional information: Anti-RbcL can be used as a cellular [compartment marker] of plastid stroma (cytoplasm in cyanobacteria) and detects RbcL protein from 31.25 fmoles. As both forms (I and II) are detected it is suitable for work with samples from Dinoflagellates, Haptophytes and Ochrophytes (diatoms, Raphidophytes, brown algae) as well as higher plants. This antibody together with Agrisera Rubisco protein standard is very suitable to quantify Rubisco in plant and algal samples.
This product can be sold containing ProClin if requested. - Background
-
Background: This antibody is especially suitable for quantifying of Rubisco in plant and algal samples.
Rubisco (Ribulose-1,5-bisphosphate carboxylase/oxygenase) catalyzes the rate-limiting step of CO2 fixation in photosynthetic organisms. It is demonstrably homologous from purple bacteria to flowering plants and consists of two protein subunits, each present in 8 copies. In plants and green algae, the large subunit (~55 kDa) is coded by the chloroplast rbcL gene, and the small subunit (15 kDa) is coded by a family of nuclear rbcS genes.
- Product Citations
-
Selected references: Mohapatra et al. (2025). An Improved Method for Isolating Intact Chloroplasts from Different Plant Species. Journal of Plant Growth Regulation, doi.org/10.1007/s00344-025-11911-4.
Rong et al. (2025). Photosynthetic and oxidative stress responses of rice seedlings exposed to benzotriazole. Sci Prog. 2025 Jul-Sep;108(3):368504251362300. doi: 10.1177/00368504251362300.
Teikari et al. (2025). Competition and interdependence define interactions of Nostoc sp. and Agrobacterium sp. under inorganic carbon limitation. NPJ Biofilms Microbiomes. 2025 Mar 8;11(1):42. doi: 10.1038/s41522-025-00675-0. (immunofluorescence)
Oh et al. (2024). Unique biogenesis and kinetics of hornwort Rubiscos revealed by synthetic biology systems. Mol Plant. 2024 Nov 2:S1674-2052(24)00352-6. doi: 10.1016/j.molp.2024.10.013.
Cui, Liu, Li, et al. (2022) The cellulose--lignin balance affects the twisted growth of Yunnan pine trunk. Authorea. October 10, 2022. DOI: 10.22541/au.166538021.18232197/v4
He, Buren, Baysal, et al. (2022) Nitrogenase Cofactor Maturase NifB Isolated from Transgenic Rice is Active in FeMo-co Synthesis. ACS Synth Biol. 2022;11(9):3028-3036. doi:10.1021/acssynbio.2c00194
Li et al. (2021). Physiological responses of Skeletonema costatum to the interactions of seawater acidification and the combination of photoperiod and temperature. Biogeosciences, 18, 1439-1449, 2021 https://doi.org/10.5194/bg-18-1439-2021
Lal et al. (2018). The Receptor-like Cytoplasmic Kinase BIK1 Localizes to the Nucleus and Regulates Defense Hormone Expression during Plant Innate Immunity. Cell Host Microbe. 2018 Apr 11;23(4):485-497.e5. doi: 10.1016/j.chom.2018.03.010.
Korotaeva et al. (2018). Effect of Heat Hardening on Expression of Genes phb3 and phb4 and Accumulation of Phb Proteins in Green Leaves of Arabidopsis thaliana. Russian Journal of Plant Physiology, 65(5), 688-696, 2018.
Lan et al (2023) UPL5 modulates WHY2 protein distribution in a Kub-site dependent ubiquitination in response to [Ca2+]cyt-induced leaf senescence. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extractsRubisco quantitation in plant and algal samples using Agrisera anti-RbcL antibody and Rubisco protein standard
Methodology: Plant samples are generally ground with liquid nitrogen in a mortar and pestle. The resulting powder is transferred to a plastic tube. Algal samples can be either concentrated by centrifugation or, preferably, by filtration onto glass fiber filters. Solubilization is performed in Agrisera protein extraction buffer (PEB, AS08 300) containing 0.1mg/mL PefaBloc SC (AEBSF) protease inhibitor (Roche). Disruption is most optimally obtained through flash freezing of the sample in liquid nitrogen alternated with thawing by sonication with a microtip. This process can be repeated depending on the toughness of the sample. The sample is adjusted to 50 mM dithiothreitol and heated to 70°C for 5 minutes. Samples are cooled and centrifuged briefly prior to electrophoresis.
Optimal quantitation is achieved using moderate sample loads per gel lane, generally 0.5 to 2.5 ug total protein, depending on the abundance of the target protein.
Electrophoresis and Immunoblotting: Once solubilized, the proteins can be separated electrophoretically in a number of systems. We obtain optimal results with the Invitrogen NuPAGE gel system using Bis-Tris 4-12% gradient gels. Proteins are separated in MES SDS running buffer according to the manufacturer’s recommendations at 200 V for 35 minutes. The gels are transferred to PVDF in the same apparatus, the SureLock XCell blot module, for 60 minutes at 30 V for a single gel or 80 minutes for a pair. Following transfer the blots are blocked with non-fat dry milk up to 10 % in TBS-T, for 1 h/RT with gentle agitation. The blot is incubated with primary antibody, usually at 1:25 000 to 1:50 000, for 1 h/RT (if extreme femtogram detection reagents are used) or in lower primary antibody dilution for less sensitivie reagents (mid picogram and lower).
For quantitation a relatively high primary antibody: target protein ratio gives more reliable results than immunoblots at low ratios of primary antibody:target protein.
The blot is washed extensively in TBS-T (twice briefly, once for 15 minutes and three times for five minutes). The blot is incubated with secondary antibody, for example goat anti-rabbit IgG horse radish peroxidase conjugated, AS09 602 (Agrisera), for 1h/RT. The blot is washed as above and developed with ECL detection reagents.Quantitation: When quantitated standards are included on the blot, the samples can be quantitated using the available software. Excellent quantitation can be obtained with images captured on the Bio-Rad Fluor-S-Max or equivalent instrument using Bio-Rad QuantityOne software. The contour tool is used to select the area for quantitation and the values are background subtracted to give an adjusted volume in counts for each standard and sample. Using above protocol linear standard curves are generated over 1-1.5 orders of magnitude range in target load. It is important to note that immunodetections usually show a strongly sigmoidal signal to load response curve, with a region of trace detection of low loads, a pseudolinear range and a region of saturated response with high loads. For immunoquantitation it is critical that the target proteins in the samples and the standard curve fall within the pseudolinear range. Our total detection range using this protocol spans over 2 orders of magnitude, but the quantifiable range is narrower.
References:
MacKenzie et al (2005). Large reallocations of carbon, nitrogen and photosynthetic reductant among phycobilisomes, photosystems and Rubisco during light acclimation in Synechococcus elongatus are constrained in cells under low environmental inorganic carbon. Arch of Microbiol. 183: 190 - 202.
Bouchard et al. (2006) UVB effects on the photosystem II-D1 protein of phytoplankton and natural phytoplankton communities. Photochem and Photobiol 82: 936-951.
Morash et al. (2007) Macromolecular dynamics of the photosynthetic system over a seasonal developmental progression in Spartina alterniflora. Can J. of Bot. 85: 476-483(8)Application example
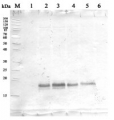
.jpg)
2 µg of total protein from (1-7) Prochlorococcus sp. extracted with Protein Extration Buffer, PEB (AS08 300), (8-11) Rubisco protein standard (AS01 017S) were separated on 4-12% NuPage (Invitrogen) LDS-PAGE and blotted 1h to PVDF. Blots were blocked immediately following transfer for 1h/RT with agitation. Blots were incubated in the primary antibody at a dilution of 1: 20 000 for 1h at room temperature with agitation. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 15 min and 3 times for 5 min in TBS-T at room temperature with agitation. Blots were incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated) diluted to 1:50 000 for 1h/RT with agitation. The blots were washed as above and developed for 5 min with ECL detection reagent according the manufacturers instructions. Images of the blots were obtained using a CCD imager (FluorSMax, Bio-Rad) and Quantity One software (Bio-Rad). There is a low level of a known RbcL cleavage product below the main band in som eof the Prochlorococcus samples.
Recommended secondary antibodies: goat anti-rabbit HRP conjugated, goat anti-rabbit ALP conjugated
Recommended chemiluminescent detection reagent: AgriseraECLBrightMethod for embedding in LR WHITE for immunolabelling using anti-RbcL antibody
Prins et al. (2008). Cysteine proteinases regulate chloroplast protein content and composition in tobacco leaves:a model for dynamic interactions with ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) vesicular bodies. J.Ex.Bot.59:1935-1950. Method described below is a courtesy of Dr Aranda
Samples must be sectioned at 1 x 1 mm. 1)Fixation: Glutaraldehyde 0.5% + paraformaldehyde 2.5% in sodium phosphate buffer 0.1M pH= 7.2, 1 hour and 30 min. 2) 3 washes in sodium phosphate buffer 0.1M pH= 7.2 (15 min. each one). 3)Dehydratation and embedding:
-35% Ethanol……30 min.
-50% Ethanol……30 min.
-70% Ethanol……30 min.
-96% Ethanol……30 min.
-100% Ethanol……30 min.
-100% Ethanol……30 min.
-100% Ethanol……30 min.-75% Ethanol + 25% LR White…… 1 hour
-50% Ethanol + 50% LR White…… 1 hour
-25% Ethanol + 75% LR White…… 1 hour
-100% LR White…………………... 1 hour
-100% LR White…………………... overnight.4) Polymerization: Oven 12-18 hours at 50ºC. For flat molds in continous flux of N2
5) Block sectioning: Ultrathin sections (60-70nm) using a ultramicrotome. Ultrathin sections should be placed on formvar coated níkel grids.
IMMUNOLABELLING
1)Blocking: Place grids with sections into 30-μL drop of blocking buffer during 30 minutes. (Blocking buffer: phosphate-buffered saline (PBS) + 5% bovine serum albumin (BSA).
2) Incubation: Incubate in drop of primary antibody, anti-RbcL (Rubisco form I and II, Agrisera) diluted 1:250 in blocking buffer. 3 hours at room temperature (could be extend to overninght).
3) Rinse: Pass the grids through a series of drops of PBS + 1%BSA. Two or three changes during 5 minutes each one.
4) Incubation: Incubate grids in 30-μL of secondary antibody gold-labelled (British BioCell International, 10nm), diluted 1:50 in blocking buffer during 1.5 hours at room temperature.
5) Rinse: Pass the grids through a series of drops of PBS + 1%BSA. Two changes during 5 minutes each one.
6) Rinse: Pass the grids through a series of drops of PBS. Two or three changes during 5 minutes each one.
7) Rinse: Pass the grids through a series of drops of water (ultra-pure water must filtered 0.2 μm) . Four to five changes during 5 minutes each one, allowed to dry for later observation.
8) Staining: The grids may be post-stained with uranyl acetate and lead citrate.
Pao et al. (2018). Lamelloplasts and minichloroplasts in Begoniaceae: iridescence and photosynthetic functioning. J Plant Res. 2018 Mar 2. doi: 10.1007/s10265-018-1020-2. (ImmunoGold)
Liang et al. (2014). Cyanophycin mediates the accumulation and storage of fixed carbon in non-heterocystous filamentous cyanobacteria from coniform mats. PLoS One. 2014 Feb 7;9(2):e88142. doi: 10.1371/journal.pone.0088142. eCollection 2014. (ImmunoGold) - Reviews:
-
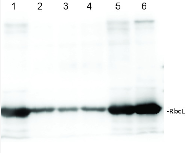
Vasundara Sundar | 2022-12-28I have used Rbcl ls antibody for western blotting at 1:10000 dilution. The results were excellent with sharp bands.