Alcohol is a standard component of transfer buffer. | 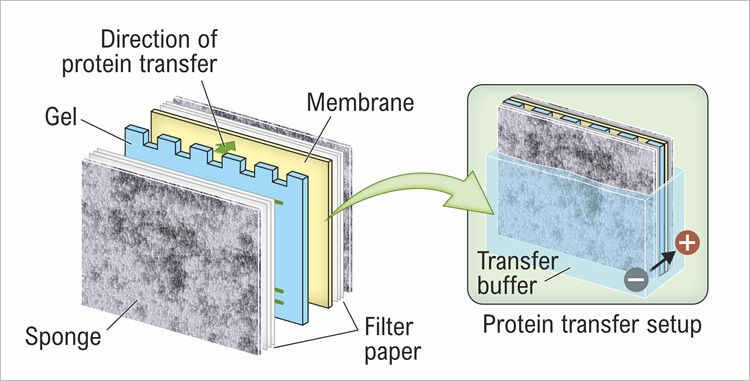 Which parameters should be considered when transferring HMW proteins? How to check if protein transfer from gel to membrane was efficient? Membrane activation before protein transfer: which one to use, methanol or ethanol? |
Latest
Same antibody, different outcome on samples from wide taxonomic range2026-02-23 Can the quality of protein transfer be checked before the blot is developed with antibodies?
2026-01-16 Is wild type a wild type?
2025-12-29 Why an antibody may detect tagged protein but not endogenous one and in some cases endogenous protein but not its tagged version?
2025-10-30 Calclulated and aparent molecular weight of detected protein is different, why?
2025-10-09 How to chose right loading control for Western blot?
2025-10-06 Is an antibody going to work in a technique I am planning to use it in?
2025-09-30 Antibody reactivity to recombinant protein,does not validate antibody specificity in endogenous sample
2025-08-20 Can aggregated antibody be still used?
2025-08-15 Blot Once, Probe Twice - when such approach can be beneficial?
2025-07-31
Archive
- February - 2026
- January - 2026
- December - 2025
- October - 2025
- September - 2025
- August - 2025
- July - 2025
- June - 2025
- May - 2025
- April - 2025
- March - 2025
- February - 2025
- January - 2025
- December - 2024
- November - 2024
- September - 2024
- July - 2024
- June - 2024
- May - 2024
- March - 2024
- February - 2024
- December - 2023
- November - 2023
- September - 2023
- July - 2023
- May - 2023
- March - 2023
- January - 2023
- December - 2022
- November - 2022
- October - 2022
- September - 2022
- August - 2022
- June - 2022
- May - 2022
- March - 2022
- February - 2022
- January - 2022
- November - 2021
- October - 2021
- August - 2021
- June - 2021
- May - 2021
- April - 2021
- March - 2021
- February - 2021
- January - 2021
- December - 2020
- November - 2020
- October - 2020
- September - 2020
- August - 2020
- July - 2020
- June - 2020
- May - 2020
- April - 2020
- March - 2020
- January - 2020
- November - 2019
- October - 2019
- March - 2019
- April - 2017
- February - 2017
- May - 2016
- February - 2014
- September - 2013
- December - 2010
