| This question has been asked during the last Western blot workshop conducted by Agrisera. Are we indeed working with a "weak antibody" or is it our protocol, which is not optimized? Do you know enough about the protein which is to be detected using an antibody? Is it low or medium expressed? In which organ or specific tissue can it be found and how to extract it? Have you first made sure, that an antibody you are going to use is a match for the protein you want to detect? If the aim is to detect a protein of low expression, which is not cytoplasmic, fractionation will concentrate this target protein to detectable levels. What protein load/well do we use? For proteins of low expressions, the load needs to be considerably higher, >25 µg/well. Have the correct membrane been used with suitable pore size and optimal buffer composition? MW of a protein we aim to detect should help us to optimize this parameter. Are used antibodies antigen affinity purified? This is of crucial importance if the aim is to detect protein of low expression. Is a suitable detection reagent used? Chromogenic detection (with alkaline phosphatase conjugated secondary antibodies) is less sensitive compare to chemiluminescent detection, which is especially recommended when target protein is not that abundant in analysed material. As you see from the list above, a concept of a "weak antibody" has to be verified by checking all parameters of Western blot procedure before drawing such conclusion. | 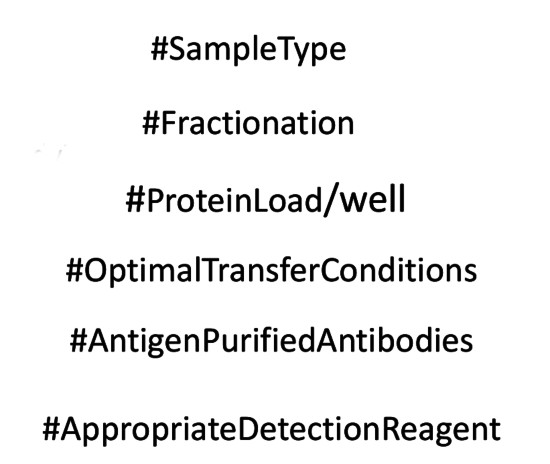 Use Agrisera Western blot Resource or send your questions to our Support Team at [email protected] or request a workshop: "Western blot - technique possible to optimize" |
Latest
Same antibody, different outcome on samples from wide taxonomic range2026-02-23 Can the quality of protein transfer be checked before the blot is developed with antibodies?
2026-01-16 Is wild type a wild type?
2025-12-29 Why an antibody may detect tagged protein but not endogenous one and in some cases endogenous protein but not its tagged version?
2025-10-30 Calclulated and aparent molecular weight of detected protein is different, why?
2025-10-09 How to chose right loading control for Western blot?
2025-10-06 Is an antibody going to work in a technique I am planning to use it in?
2025-09-30 Antibody reactivity to recombinant protein,does not validate antibody specificity in endogenous sample
2025-08-20 Can aggregated antibody be still used?
2025-08-15 Blot Once, Probe Twice - when such approach can be beneficial?
2025-07-31
Archive
- February - 2026
- January - 2026
- December - 2025
- October - 2025
- September - 2025
- August - 2025
- July - 2025
- June - 2025
- May - 2025
- April - 2025
- March - 2025
- February - 2025
- January - 2025
- December - 2024
- November - 2024
- September - 2024
- July - 2024
- June - 2024
- May - 2024
- March - 2024
- February - 2024
- December - 2023
- November - 2023
- September - 2023
- July - 2023
- May - 2023
- March - 2023
- January - 2023
- December - 2022
- November - 2022
- October - 2022
- September - 2022
- August - 2022
- June - 2022
- May - 2022
- March - 2022
- February - 2022
- January - 2022
- November - 2021
- October - 2021
- August - 2021
- June - 2021
- May - 2021
- April - 2021
- March - 2021
- February - 2021
- January - 2021
- December - 2020
- November - 2020
- October - 2020
- September - 2020
- August - 2020
- July - 2020
- June - 2020
- May - 2020
- April - 2020
- March - 2020
- January - 2020
- November - 2019
- October - 2019
- March - 2019
- April - 2017
- February - 2017
- May - 2016
- February - 2014
- September - 2013
- December - 2010
