Plant organelle/membrane isolation
- Arabidopsis lumen extraction
- Arabidopsis thylakoid extraction
- BBY preparation
- Chlorophyll measurements
- Intact chloroplast isolation method
- Mitochondrial fraction
- Nuclear fraction
- Plasma membrane fraction
- PSII RC extraction for cryo-EM
- Thylakoid extraction
- Vacuol isolation
- Collection of articles

- Diatom protein extraction
- Extraction of leaf proteins
- Phenol protein extraction
- Ponceau membrane staining
- Protein extraction from grasses
- TCA acetone precipitation method
- Western blot protocol
- Western blot video tutorial
- Western blot troubleshooting

- Western blot using IgY
- Western blot in denatured conditions (urea gel)

- Peptide neutralization/competition assay
- Quantitative Western blot
- Quantitative Western blot video tutorial
- Simultaneous Western blot
- Anti-KLH antibody removal

- Dot blot
- ELISA
- Immunohistochemistry
- Immunoprecipitation
- Immunoprecipitation/IgY
- Meiotic staining
- Yolk delipidation
Technical information
Antibody typesPurification
- Antibody purification
- Antibody purification - small amount of protein
- Elution of antibodies from affinity columns
- IgY purification methods
- Protein purification using antibodies
Protocols > Diatom Protein ExtractionProcedure
ReferenceWu et al. (2011). Distinctive photosystem II photoinactivation and protein dynamics in marine diatoms. Plant Physiol. 156(4):2184-95.
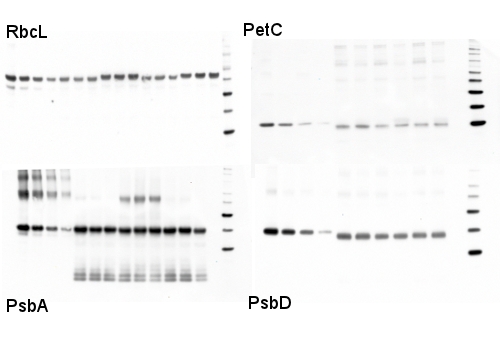
Western blot detection of photosynthetic proteins from diatoms
1 µg of total protein from various diatom cell pellets extracted with LiDS protein extraction buffer PEB (Agrisera) were separated for 40 min on 4-20% gradient Invitrogen NuPAGE gels and blotted 1h to PVDF. Blots were blocked with ECL Advance blocking agent for 1 h at room temperature (RT) with agitation. Blot was incubated in the primary antibody at a dilution of 1: 10 000 to 1:25 000 (depending upon the antibody) for 1 h at RT with agitation. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 15 min and 3 times for 5 min in TBS-T at RT with agitation. Blot was incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, from Agrisera, AS09 602 ) diluted to 1:25 000 in blocking buffer for 1h at RT with agitation. The blot was washed as above and developed for 5 min with ECL Advance according to the manufacturer's instructions. Exposure time was 30-60 seconds with a BioRad VersaDoc CCD Imager. High dilutions of primary and secondary antibodies are used to extend the pseudo-linear range of the signal-to-target ratio, by limiting steric interference which can lower signal-to-target ratio under excess antibody levels. Protein Extraction – Diatoms Although we have started incorporating an automatic, mechanical "bead-beater" in the lab for our samples (so fast! so consistent!), every once in a while we find it necessary to go back to our tried and true "soni-thaw" method (sonication tip submerged into flash frozen samples until they reach the consistency of a green slushie). In both instances we always do a test run with a new species, where cells are exposed to increasing levels of disruption (ex: 1x-8x 30 second runs on the bead-beater or 1x-4x soni-thaw rounds). The samples are assayed for total protein quantity and then verified by Western Blotting for degradation. It sounds like a long process, but with practice you can get the ideal disruption protocol - that maximizes protein release while minimizing degradation - in a day and a half. When growing pure cultures, we often just pellet the material by centrifugation, sometimes with the addition of the flocking agent Pluronic Acid F-68. When using mixed media samples (where ideal centrifugation values differ between cell types) or environmental samples (where a centrifuge is often a missing luxury) we resort to vacuum filtration of liquid onto binder-free (BF) filters. The binding agents used in the glass filters tend to gum up mechanical disruption methods, so it is vital to order the correct, binder-free filter for downstream protein extraction.
|