Prof. Rossana Henriques

Dr. Rossana Henriques is the Paddy O'Keeffe Senior Lecturer (Associate Professor) in Plant Genetics at the School of Biological, Earth and Environmental Sciences at University College Cork (UCC), a role she has held since 2018. Prior to this, she was a junior Principal Investigator at the Centre for Research in Agricultural Genomics (CRAG) in Barcelona, and a Research Associate in Professor Nam-Hai Chua's lab at The Rockefeller University in New York. She was a postdoctoral fellow at Royal Holloway College, University of London, and completed her PhD through a collaboration between the University of Lisbon and the Max-Planck Institute in Cologne.
- Please tell us about yourself and your research/institution.I'm interested in the regulation of plant growth, and how that connects to seasonal conditions. I.e. how plants measure time and use that information to control their growth patterns. More specifically, I study how plants use day length and temperature cues to modulates their growth and development responses. At University College Cork, where I've been for the last 7 years, we have continued the research I was previously conducting at the Centre for Research in Agricultural Genomics, examining how the TOR pathway regulates growth responses. Initially, we used Arabidopsis, but we are expanding the studies to include forage grasses and legumes.
We are studying how plants grow under short and long day conditions, and how that correlates with temperature. Considering that climate change is increasing the average temperature on Earth, leading to extreme events, like periods of drought or flooding, we have also done research assessing how a higher temperature, and some abiotic stress factors, impact growth responses. Beyond that, we are examining what happens with components of the TOR pathway when the environmental conditions are not optimal. Besides TOR, we have also done some work with long non-coding RNAs, which connects to both abiotic stress responses and developmental questions, like flowering time regulation.
- What motivated you to get into plant science?My degree is in biology, but I knew that I wasn't going to be a zoologist or ecologist. I've always been more interested in molecular biology and biochemistry, but from a plant perspective. Many signaling pathways are well-studied and understood in animal research, but not in plant science. I've always been keen on trying to see how we could answer questions that were already well-established in animal research, using plants. So, developing new methodologies and setting up new strategies, to address e.g. how plant proteins accumulate. I find it very interesting how plants do things, and what the molecular mechanisms that underlie their responses are. From the plant's perception of what is going on, and how that translates into specific decisions at a cellular level, to how this impacts organs, and ultimately the organism itself. Plants are very plastic, and have many particular abilities. Any plant cell could be a stem cell, given the right conditions. This is something that has recently gained traction in animal research, while in plant research, we've been doing tissue culture, and making leaves into embryos since the '60-70s. Something I've always liked with plants, is the ability to work with different tissues and address a lot of different questions. There is also the fact that without plants, there really is no life on this planet. A lot of people don't realize how vital plants are to food security and our life.
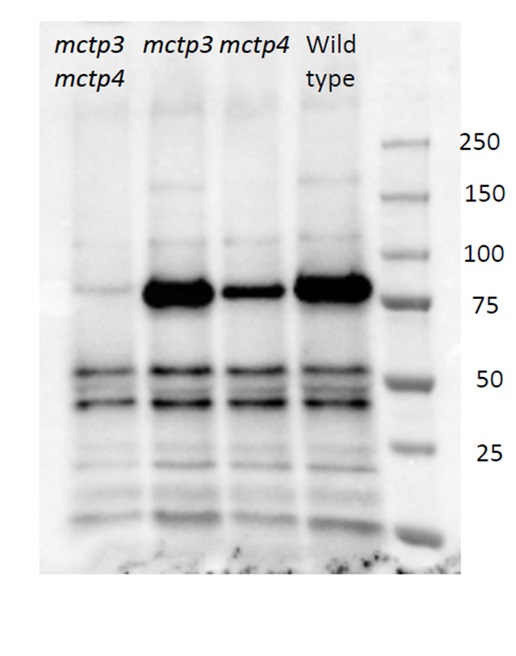
- How have you used (Agrisera) antibodies in your research?I've been collaborating with Agrisera for several years, and I think it's brilliant that there is a company that really provides researchers in plant science with appropriate tools. For a long time, I struggled with working with antibodies that were published in research for animals. All the Western blots, immunoprecipitations and data from animal publications looked brilliant, but when you tried to replicate it with plant material, many of those things didn't work. I was really happy when we started our collaboration and interaction with Agrisera. There was finally a company that really put their resources and efforts into working for the plant science community.
I am very grateful for Agrisera's strong collaboration with scientists, and in our specific case, the development of several antibodies for the TOR pathway. We wouldn't have been able to do the work we have done without your antibodies. It allowed us to do a lot of research in characterizing the TOR pathway. I think this is what makes Agrisera a special company, for me as a plant biologist.
I would also like to highlight the fact Agrisera is always interested in new antibody suggestions from the plant science community, and the fact that some of us can try the antibodies, validate them, and determine the best conditions for detection. It's very useful that you have the data from researchers, with whom you've collaborated, presented on your website. You show the actual Western blots, and the conditions for them. I really appreciate that this allows others to follow all the instructions on how to use your antibodies.
To me, another important aspect is that cross-reactivity is specified for your antibodies. Arabidopsis has been very good to us, in terms of a model species, allowing us to ask a lot of questions, and develop common tools. But with broad antibody species reactivity, we can allow ourselves to ask other questions, and move on to other species, connected to food security.
Links
• Prof. Rossana Henriques, University College Cork
• Agrisera antibodies to proteins involved in Plant Signal Transduction
• Agrisera antibodies to proteins involved in environmental stress response